| Names | |
|---|---|
| IUPAC name Rhenium(III) chloride | |
| Other names Rhenium trichloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) |
|
| ECHA InfoCard | 100.033.610 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | ReCl3 |
| Molar mass | 292.57 g/mol |
| Appearance | red, crystalline, nonvolatile solid |
| Density | 4800 kg/m |
| Melting point | N/A |
| Boiling point | 500 °C (932 °F; 773 K) (decomposes) |
| Solubility in water | hydrolyzes to form Re2O3xH2O. |
| Structure | |
| Crystal structure | Rhombohedral, hR72 |
| Space group | R-3m, No. 166 |
| Molecular shape | (trimeric solid and in solution) (dimeric in acetic acid) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Corrosive (C) |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
| Other anions | Rhenium tribromide Rhenium triiodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Trirhenium nonachloride is a compound with the formula ReCl3, sometimes also written Re3Cl9. It is a dark red hygroscopic solid that is insoluble in ordinary solvents. The compound is important in the history of inorganic chemistry as an early example of a cluster compound with metal-metal bonds. It is used as a starting material for synthesis of other rhenium complexes.
Structure and physical properties
As shown by X-ray crystallography trirhenium nonachloride consists of Re3Cl12 subunits that share three chloride bridges with adjacent clusters. The interconnected network of clusters forms sheets. Around each Re center are seven ligands, four bridging chlorides, one terminal chloride, and two Re-Re bonds.
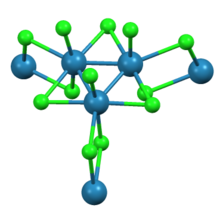
The hydrate is molecular with the formula Re3Cl9(H2O)3.
The heat of oxidation is evaluated according to the equation:
- 1/3 Re3Cl9 + 4 OH + 2 OCl → ReO4 + 2 H2O + 5Cl
The enthalpy for this process is 190.7 ± 0.2 kcal/mol.
Preparation and reactions
The compound was discovered in 1932. Trirhenium nonachloride is efficiently prepared by thermal decomposition of rhenium pentachloride or hexachlororhenic(IV) acid:
- 3 ReCl5 → Re3Cl9 + 3 Cl2
If the sample is vacuum sublimed at 500 °C, the resulting material is comparatively unreactive. The partially hydrated material such as Re3Cl9(H2O)4 can be more useful synthetically. Other synthetic methods include treating rhenium with sulfuryl chloride. This process is sometimes conducted with the addition of aluminium chloride. It is also obtained by heating Re2(O2CCH3)4Cl2 under HCl:
- 3/2 Re2(O2CCH3)4Cl2 + 6 HCl → Re3Cl9 + 6 HO2CCH3
Reaction of the tri- and pentachlorides gives rhenium tetrachloride:
- 3 ReCl5 + Re3Cl9 → 6 ReCl4
References
- Cotton, F. A.; Walton, R. A. "Multiple Bonds Between Metal Atoms" Oxford (Oxford): 1993. ISBN 0-19-855649-7.
- ^ Colton, R. Chemistry of rhenium and technetium. 965.
- Irmler, Manfred; Meyer, Gerd (1987). "Rhenium trichloride, ReCl3, and its 5/3-hydrate synthesis, crystal structure, and thermal expansion". Zeitschrift für Anorganische und Allgemeine Chemie. 552 (9): 81–89. doi:10.1002/zaac.19875520908.
- Geilnann, W.; Wriuce, F. W.; Biltz. W.: Nachr. Ges. Wiss. Gottingen 1932, 579.
- Lincoln, R.; Wilkinson, G. (1980). "Trirhenium Nonachloride". Inorg. Synth. 20: 44. doi:10.1002/9780470132517.ch12. ISBN 978-0-470-13251-7.
- Irmler, Manfred; Meyer, Gerd (1987). "Rhenium trichloride, ReCl3, and its 5/3-hydrate synthesis, crystal structure, and thermal expansion". Zeitschrift für Anorganische und Allgemeine Chemie. 552 (9): 81–89. doi:10.1002/zaac.19875520908.
| Rhenium compounds | |||||
|---|---|---|---|---|---|
| Rhenium(0) |
| ||||
| Rhenium(I) |
| ||||
| Rhenium(II) | |||||
| Rhenium(III) | |||||
| Rhenium(IV) | |||||
| Rhenium(V) | |||||
| Rhenium(VI) | |||||
| Rhenium(VII) |
| ||||