| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C15H15Sc |
| Molar mass | 240.241 g·mol |
| Melting point | 240 °C (513 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
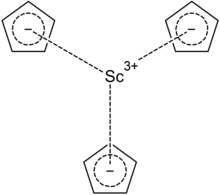
Scandocene is an organoscandium compound with the chemical formula Sc(C5H5)3. It is a straw-colored crystal and can be obtained by reacting anhydrous scandium(III) chloride and sodium cyclopentadienide in tetrahydrofuran. If scandium(III) fluoride and magnesocene are reacted as raw materials, a mixture of scandium(III) fluoride and scandocene will be obtained. It decomposes when exposed to water to produce cyclopentadiene and scandium(III) hydroxide.
References
- ^ G. Wilkinson, J. M. Birmingham (Dec 1954). "CYCLOPENTADIENYL COMPOUNDS OF Sc, Y, La, Ce AND SOME LANTHANIDE ELEMENTS". Journal of the American Chemical Society. 76 (23): 6210. doi:10.1021/ja01652a114. ISSN 0002-7863. Archived from the original on 2021-07-16. Retrieved 2020-12-09.
- Frank Bottomley, Daniel E. Paez, Peter S. White (Aug 1985). "The reaction of scandium trifluoride with cyclopentadienyl salts and the crystal and molecular structure of the trimer of fluorodicyclopentadienylscandium". Journal of Organometallic Chemistry. 291 (1): 35–41. doi:10.1016/0022-328X(85)80200-X. Archived from the original on 2018-06-27. Retrieved 2020-12-09.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
| Scandium compounds | |
|---|---|
| Salts and covalent derivatives of the cyclopentadienide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||