| Revision as of 22:42, 16 December 2012 editMakecat-bot (talk | contribs)103,877 editsm r2.7.3) (Robot: Adding ru:Бигуаниды← Previous edit | Revision as of 05:44, 15 March 2013 edit undoAddbot (talk | contribs)Bots2,838,809 editsm Bot: Migrating 10 interwiki links, now provided by Wikidata on d:q142880Next edit → | ||
| Line 80: | Line 80: | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Revision as of 05:44, 15 March 2013
| This article may have too many section headers. Please help consolidate the article. (June 2012) (Learn how and when to remove this message) |
| This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. Please help improve this article by introducing more precise citations. (January 2011) (Learn how and when to remove this message) |

| |

| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 507183 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.229 |
| EC Number |
|
| Gmelin Reference | 240093 |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2H7N5 |
| Molar mass | 101.113 g·mol |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
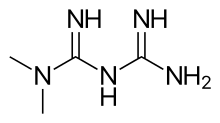

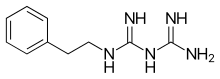
Biguanide can refer to a molecule, or to a class of drugs based upon this molecule. Biguanides can function as oral antihyperglycemic drugs used for diabetes mellitus or prediabetes treatment. They are also used as antimalarial drugs.
The disinfectant polyaminopropyl biguanide (PAPB) features biguanide functional groups.
Examples
Examples of biguanides:
- Metformin - widely used in treatment of diabetes mellitus type 2
- Phenformin - withdrawn from the market in most countries due to toxic effects
- Buformin - withdrawn from the market due to toxic effects
- Proguanil - an antimalarial drug.
History
Galega officinalis (French lilac) was used for diabetes treatment in traditional medicine for centuries. In the 1920s, guanidine compounds were discovered in Galega extracts. Animal studies showed that these compounds lowered blood glucose levels. Some less toxic derivatives, synthalin A and synthalin B, were used for diabetes treatment, but after the discovery of insulin they were forgotten for the next several decades. Biguanides were reintroduced into Type 2 diabetes treatment in the late 1950s. Initially phenformin was widely used, but its potential for sometimes fatal lactic acidosis resulted in its withdrawal from pharmacotherapy in most pharmacopeias (in the U.S. in 1977). Metformin has a much better safety profile, and it is the principal biguanide drug used in pharmacotherapy worldwide.
Pharmacotherapy
Biguanides do not affect the output of insulin, unlike other hypoglycemic agents such as sulfonylureas and meglitinides. Therefore, not only are they effective in Type 2 diabetics but they can also be effective in Type 1 patients in concert with insulin therapy.
Mechanism of action
The mechanism of action of biguanides is not fully understood. Mainly used in Type II Diabetes, Metformin is considered to increase insulin sensitivity in vivo, resulting in reduced plasma glucose concentrations, increased glucose uptake, and decreased gluconeogenesis.
However, in hyperinsulinemia, biguanides can lower fasting levels of insulin in plasma. Their therapeutic uses derive from their tendency to reduce gluconeogenesis in the liver, and, as a result, reduce the level of glucose in the blood. Biguanides also tend to make the cells of the body more willing to absorb glucose already present in the blood stream, and there again reducing the level of glucose in the plasma.
Side effects and toxicity
The most common side effect is diarrhea and dyspepsia, occurring in up to 30% of patients. The most important and serious side effect is lactic acidosis, therefore metformin is contraindicated in renal insufficiency. Renal functions should be assessed before starting metformin. Phenformin and buformin are more prone to cause acidosis than metformin; therefore they have been practically replaced by it. However, when metformin is combined with other drugs (combination therapy), hypoglycemia and other side effects are possible.
References
- Rang et al., Pharmacology, 5th Edition, 2003, p 388
| Antiparasitics – antiprotozoal agents – Chromalveolata antiparasitics (P01) | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alveo- late |
| ||||||||||||||||||||||||||||||||||||||||||||||
| Stramen- opile | |||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||