| |
| Names | |
|---|---|
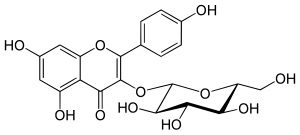
| IUPAC name 3-(β-D-Glucopyranosyloxy)-4′,5,7-trihydroxyflavone | |
| Systematic IUPAC name 5,7-Dihydroxy-2-(4-hydroxyphenyl)-3-{oxy}-4H-1-benzopyran-4-one | |
| Other names
Astragaline asragalin kaempferol-3-glucoside Kaempferol 3-glucoside Kaempferol 3-O-glucoside Kaempferol-3-O-glucoside Kaempferol-3-D-glucoside Kaempferol-3-beta-monoglucoside Kaempferol 3-O-β-D-glucopyranoside | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 100568 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.128.596 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H20O11 |
| Molar mass | 448.380 g·mol |
| Density | 1.791 g/mL |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Astragalin is a chemical compound. It can be isolated from Phytolacca americana (the American pokeweed) or in the methanolic extract of fronds of the fern Phegopteris connectilis. It is also found in wine.
Astragalin is a 3-O-glucoside of kaempferol.
References
- Adam, Klaus-Peter (1999). "Phenolic constituents of the fern Phegopteris connectilis". Phytochemistry. 52 (5): 929–934. Bibcode:1999PChem..52..929A. doi:10.1016/S0031-9422(99)00326-X.
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |