| |
| Names | |
|---|---|
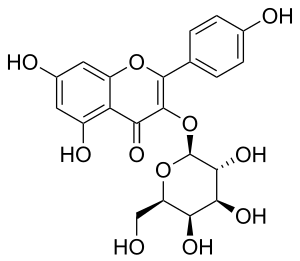
| IUPAC name 3-(β-D-Galactopyranosyloxy)-4′,5,7-trihydroxyflavone | |
| Systematic IUPAC name 5,7-Dihydroxy-2-(4-hydroxyphenyl)-3-{oxy}-4H-1-benzopyran-4-one | |
| Other names
Kaempferol 3-O-β-D-galactoside Kaempferol-3-O-galactoside | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H20O11 |
| Molar mass | 448.37 g/mol |
| Density | 1.791 g/mL |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Trifolin is a chemical compound. It is the kaempferol 3-galactoside. It can be found in Camptotheca acuminata, in Euphorbia condylocarpa or in Consolida oliveriana.
Kaempferol 3-O-galactosyltransferase is an enzyme that catalyzes the chemical reaction:
UDP-galactose + kaempferol → UDP + kaempferol 3-O-beta-D-galactoside (trifolin). It can also be found in seedlings of Vigna mungo.
References
- Li, S.; Zhang, Z.; Cain, A.; Wang, B.; Long, M.; Taylor, J. (2005). "Antifungal Activity of Camptothecin, Trifolin, and Hyperoside Isolated fromCamptotheca acuminata". Journal of Agricultural and Food Chemistry. 53 (1): 32–37. doi:10.1021/jf0484780. PMID 15631505.
- Roshchin, Y. V. (1977). "Trifolin from Euphorbia condylocarpa". Chemistry of Natural Compounds. 13 (4): 481–482. doi:10.1007/BF00565849. S2CID 4813721.
- Díaz, J. S.; Carmona, A.; Torres, F.; Quintana, J.; Estévez, F.; Herz, W. (2008). "Cytotoxic Activities of Flavonoid Glycoside Acetates from Consolida oliveriana". Planta Medica. 74 (2): 171–174. doi:10.1055/s-2008-1034278. PMID 18214815. S2CID 1783362.
- Miller, K. D.; Guyon, V.; Evans, J. N.; Shuttleworth, W. A.; Taylor, L. P. (1999). "Purification, Cloning, and Heterologous Expression of a Catalytically Efficient Flavonol 3-O-Galactosyltransferase Expressed in the Male Gametophyte of Petunia hybrida". Journal of Biological Chemistry. 274 (48): 34011–34019. doi:10.1074/jbc.274.48.34011. PMID 10567367.
- "Partial Purification and Some Properties of Flavonol 3-O-Glycosyltransferases from Seedlings of Vigna mungo, with Special Reference to the Formation of Kaempferol 3-O-Galactoside and 3-O-Glucoside". Plant and Cell Physiology. 34 (2): 329–335. March 1993. doi:10.1093/oxfordjournals.pcp.a078424. ISSN 1471-9053.
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |