| |
| Names | |
|---|---|
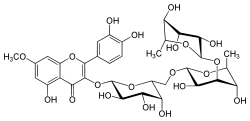
| IUPAC name 3′,4′,5-Trihydroxy-7-methoxy-2-flavone | |
| Systematic IUPAC name (4S,4R,4S,4R,4R,7R,7R,7R,7S,7S,9S,9R,9R,9R,9S)-1,1,2,4,4,4,7,7,9,9,9-Undecahydroxy-2-methoxy-7,9-dimethyl-2H-3,6,8-trioxa-2(2,3)-benzopyrana-4(2,6),7(2,4),9(2)-tris(oxana)-1(1)-benzenanonaphan-2-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C34H42O20 |
| Molar mass | 770.68 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Xanthorhamnin is a chemical compound. It can be isolated from buckthorn berries (Rhamnus catharticus).
The aglycone of xanthorhamnin is rhamnetin.
References
- "The Color of Art Pigment Database: Pigment Yellow, PY". Art is Creation.
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |