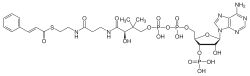
| |
| Names | |
|---|---|
| IUPAC name 3′-O-Phosphonoadenosine 5′-sulfanyl}ethyl)amino]-3-oxopropyl}amino)-4-oxobutyl dihydroxen diphosphate] | |
| Systematic IUPAC name methyl (3R)-3-hydroxy-2,2-dimethyl-4-({3-sulfanyl}ethyl)amino]-3-oxopropyl}amino)-4-oxobutyl dihydrogen diphosphate | |
| Other names
Cinnamoyl-coa (E)-cinnamoyl-CoA Coenzyme A, S-(3-phenyl-2-propenoate) (E)-benzylideneacetyl-CoA 3-phenylacryloyl-CoA | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C30H42N7O17P3S |
| Molar mass | 897.68 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Cinnamoyl-coenzyme A is an intermediate in the phenylpropanoid metabolic pathway.
Enzymes using cinnamoyl-coenzyme A
- Cinnamoyl-CoA reductase, an enzyme that catalyzes the chemical reaction cinnamaldehyde + CoA + NADP+ → cinnamoyl-CoA + NADPH + H+
- Pinosylvin synthase, an enzyme that catalyzes the chemical reaction 3 malonyl-CoA + cinnamoyl-CoA → 4 CoA + pinosylvin + 4 CO2
- Cinnamoyl-CoA:phenyllactate CoA-transferase, an enzyme that catalyzes the chemical reaction (E)-cinnamoyl-CoA + (R)-phenyllactate → (E)-cinnamate + (R)-phenyllactyl-CoA
References
| Types of hydroxycinnamic acids | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglycones |
| ||||||||||||||||
| Esters |
| ||||||||||||||||
| Oligomeric forms |
| ||||||||||||||||
| Conjugates with coenzyme A (CoA) | |||||||||||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |