| |
| Names | |
|---|---|
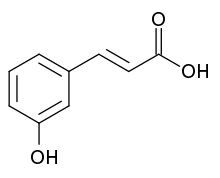
| Preferred IUPAC name (2E)-3-(3-Hydroxyphenyl)prop-2-enoic acid | |
| Other names
meta-Coumaric acid 3-Hydroxycinnamic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.742 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H8O3 |
| Molar mass | 164.16 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
m-Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers of coumaric acid – o-coumaric acid, m-coumaric acid, and p-coumaric acid – that differ by the position of the hydroxy substitution of the phenyl group.
m-Coumaric acid can be found in vinegar.
References
| Types of hydroxycinnamic acids | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglycones |
| ||||||||||||||||
| Esters |
| ||||||||||||||||
| Oligomeric forms |
| ||||||||||||||||
| Conjugates with coenzyme A (CoA) | |||||||||||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |