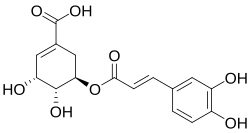
| |
| Names | |
|---|---|
| Preferred IUPAC name (3R,4R,5R)-3-{oxy}-4,5-dihydroxycyclohex-1-ene-1-carboxylic acid | |
| Other names Dattelic acid; 5-O-Caffeoylshikimic acid; trans-5-O-Caffeoylshikimic acid; 5-Caffeoylshikimic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H16O8 |
| Molar mass | 336.296 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Dactylifric acid (also known as dattelic acid or 5-O-caffeoylshikimic acid) is an ester derived from caffeic acid and shikimic acid. It and its isomers are enzymic browning substrates found in dates (Phoenix dactylifera fruits).
Some older sources identify dactylifric acid as 3-O-caffeoylshikimic acid.
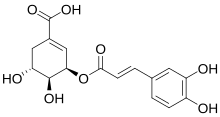
References
- "5-O-Caffeoylshikimic acid". CAS Common Chemistry.
- Fukuoka, Masamichi (1982). "Chemical and toxicological studies on bracken fern, Pteridium aquilinum var. Latiusculum. VI. Isolation of 5-O-caffeoylshikimic acid as an antithiamine factor". Chemical and Pharmaceutical Bulletin. 30 (9): 3219–3224. doi:10.1248/cpb.30.3219. PMID 6926750.
5-O-Caffeoylshikimic acid (dactylifric acid) was isolated...
- ^ Ziouti, A.; Modafar, C.; Fleuriet, A.; Boustani, S.; Macheix, J. J. (1996). "Phenolic compounds in date palm cultivars sensitive and resistant to Fusarium oxysporum". Biologia Plantarum. 38 (3): 451–457. doi:10.1007/BF02896679. S2CID 38035795.
5-caffeoylshikimic acid (dactylifric acid) and its positional isomers (3-caffeoylshikimic acid and 4-caffeoylshikimic acid)...
- "Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity" (PDF).
Trivial Name: Dactylifric acid ... Current Interpretation with IUPAC numbering: 5-O-Caffeoylshikimic acid
- ^ Maier, V. P.; Metzler, D. M.; Huber, A. F. (1964). "3-O-Caffeoylshikimic acid (dactylifric acid) and its isomers, a new class of enzymic browning substrates". Biochemical and Biophysical Research Communications. 14 (2): 124–128. doi:10.1016/0006-291x(64)90241-4. PMID 5836492.
External links
| Types of hydroxycinnamic acids | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglycones |
| ||||||||||||||||
| Esters |
| ||||||||||||||||
| Oligomeric forms |
| ||||||||||||||||
| Conjugates with coenzyme A (CoA) | |||||||||||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |