| |

| |
| Names | |
|---|---|
| IUPAC name Nickel(2+) diphosphate | |
| Other names Nickel(III) phosphate, nickel diphosphate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.755 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Ni3(PO4)2 |
| Molar mass | 366.022924 g/mol |
| Density | 4.38 g/cm |
| Solubility product (Ksp) | 4.74×10 |
| Structure | |
| Crystal structure | Monoclinic, mP26 |
| Space group | P21/c, No. 14 |
| Lattice constant | a = 0.58273 nm, b = 0.46964 nm, c = 1.01059 nmα = 90°, β = 91.138°, γ = 90° |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H317, H334, H372, H410 |
| Precautionary statements | P203, P260, P261, P264, P270, P272, P273, P280, P284, P302+P352, P304+P340, P318, P319, P321, P333+P317, P342+P316, P362+P364, P391, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Safety data sheet (SDS) | www.fishersci.com |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Nickel(II) phosphate is an inorganic compound with the formula Ni3(PO4)2. It is a mint green paramagnetic solid that is insoluble in water.
Hydrated nickel(II) phosphate
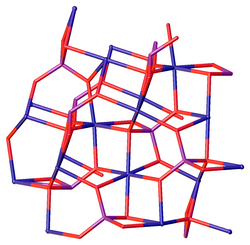
The hydrate Ni3(PO4)2·8(H2O) is a light green solid, which can be prepared by hydrothermal synthesis and also occurs as the mineral arupite. It features octahedral Ni centers, which are bound to water and phosphate.
References
- John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–189. ISBN 978-1138561632.
- McMurdie, Howard F.; Morris, Marlene C.; Evans, Eloise H.; Paretzkin, Boris; Wong-Ng, Winnie; Zhang, Yuming; Hubbard, Camden R. (2013). "Standard X-Ray Diffraction Powder Patterns from the JCPDS Research Associateship". Powder Diffraction. 2 (1): 41–52. Bibcode:1987PDiff...2...41M. doi:10.1017/S0885715600012239. S2CID 251057066.
- Calvo, Crispin; Faggiani, Romolo (1975). "Structure of Nickel Orthophosphate". Canadian Journal of Chemistry. 53 (10): 1516–1520. doi:10.1139/v75-210.
- Perry, Dale L. (18 May 2011). Handbook of Inorganic Compounds, Second Edition. CRC Press. p. 292. ISBN 978-1-4398-1462-8.
- Shouwen, Jin; Wang, Daqi; Gao, Xinjun; Wen, Xianhong; Zhou, Jianzhong (2008). "Poly[octaaquadi-μ-phosphato-trinickel(II)]". Acta Crystallographica Section E. 64 (Pt 1): m259. Bibcode:2008AcCrE..64M.259S. doi:10.1107/S1600536807067050. PMC 2915172. PMID 21200596.
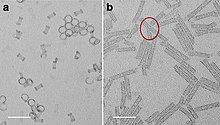
- Ni, Bing; Liu, Huiling; Wang, Peng-Peng; He, Jie; Wang, Xun (2015). "General synthesis of inorganic single-walled nanotubes". Nature Communications. 6: 8756. Bibcode:2015NatCo...6.8756N. doi:10.1038/ncomms9756. PMC 4640082. PMID 26510862.
| Nickel compounds | |
|---|---|
| Nickel(0) | |
| Nickel(II) | |
| Nickel(III) | |
| Nickel(IV) | |
| Phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||