| |

| |
| Names | |
|---|---|
| Other names Potassium tetracyanonickelate(II); dipotassium tetracyanonickelate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.034.605 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
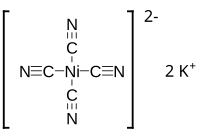
| Chemical formula | K2Ni(CN)4 |
| Appearance | yellow solid |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H300, H310, H330, H410 |
| Precautionary statements | P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P302+P350, P304+P340, P310, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Potassium tetracyanonickelate (IUPAC: Potassium tetracyanido nickelate(II)) is the inorganic compound with the formula K2Ni(CN)4. It is usually encountered as the monohydrate but the anhydrous salt is also known. Both are yellow, water-soluble, diamagnetic solids. The salt consists of potassium ions and the tetracyanonickelate coordination complex, which is square planar. The anions are arranged in a columnar structure with Ni---Ni distances of 4.294 Å, which is well beyond the sum of the van der Waals radius of the nickel cation. This columnar structure resembles those of the other anions of the heavy congeners of the group 10 metals (M = Pd, Pt).
Preparation

Potassium tetracyanonickelate is prepared by treating aqueous solutions of nickel(II) salts with potassium cyanide. The synthesis is often conducted stepwise, beginning with precipitating solid nickel dicyanide coordination polymer. This route allows removal of excess potassium salts:
- Ni + 2 KCN → Ni(CN)2 + 2 K
- Ni(CN)2 + 2 KCN → K2
This procedure yields the monohydrate. That solid dehydrates at 100 °C.
Reactions
The N-terminus of the cyanide ligand is basic and nucleophilic. The complex binds four equivalents of boron trifluoride:
- K2 + 4 BF3 → K2
Cyanide is a sufficient pi-acceptor ligand to allow reduction of K2Ni(CN)4 to the Ni(0) derivative. Thus, potassium in anhydrous ammonia affords the tetraanionic, tetrahedral Ni(0) derivative .
- K2 + 2 K → K4
An intermediate in this conversion is K4, which features an Ni-Ni bond.
References
- Vannerberg, Nils Gosta (1964). "The Crystal Structure of K2Ni(CN)4" (PDF). Acta Chemica Scandinavica. 18 (10): 2385–2391. doi:10.3891/acta.chem.scand.18-2385. Retrieved 29 April 2016. ICSD number 24099
- Fernelius, W. C.; Burbage, Joseph J. (1946). "Potassium Tetracyanonickelate(II)". Inorganic Syntheses. Inorganic Syntheses. Vol. 2. pp. 227–228. doi:10.1002/9780470132333.ch73. ISBN 9780470132333.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 426. ISBN 978-0-08-037941-8.
- Jarchow, O.; Schulz, H.; Nast, R. (1970). "Structure of the Anion in Solid K4[Ni2(CN)6]". Angewandte Chemie International Edition in English. 9: 71. doi:10.1002/anie.197000711.
| Nickel compounds | |
|---|---|
| Nickel(0) | |
| Nickel(II) | |
| Nickel(III) | |
| Nickel(IV) | |
| Salts and covalent derivatives of the cyanide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||