
| |
| |
| Names | |
|---|---|
| Systematic IUPAC name Nickel(2+) diacetate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) |
|
| ECHA InfoCard | 100.006.147 |
| EC Number |
|
| PubChem CID | |
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H6NiO4 |
| Molar mass | 176.781 g·mol |
| Appearance | Mint-green Solid |
| Odor | slight acetic acid |
| Density | 1.798 g/cm (anhydrous) 1.744 g/cm (tetrahydrate) |
| Melting point | decomposes when heated |
| Solubility in water | Easily soluble in cold water, hot water |
| Solubility | Soluble in methanol insoluble in diethyl ether, n-octanol |
| Magnetic susceptibility (χ) | +4,690.0·10 cm/mol |
| Structure | |
| Crystal structure | monoclinic |
| Space group | P21/c |
| Lattice constant | a = 4.764, b = 11.771, c = 8.425 Åα = 90°, β = 93.6°, γ = 90°tetrahydrate |
| Lattice volume (V) | 471.5 |
| Formula units (Z) | 2 |
| Coordination geometry | distorted octahedral |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 350 mg/kg (rat, oral) 410 mg/kg (mouse, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
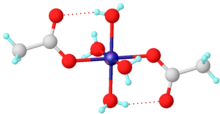
Nickel(II) acetate is the name for the coordination compounds with the formula Ni(CH3CO2)2·x H2O where x can be 0, 2, and 4. The mint-green tetrahydrate Ni(CH3CO2)2·4 H2O is most common. It is used for electroplating.
Synthesis and structure
The compound can be prepared by treating nickel or nickel(II) carbonate with acetic acid:
- NiCO3 + 2 CH3CO2H + 3 H2O → Ni(CH3CO2)2·4 H2O + CO2
The mint-green tetrahydrate has been shown by X-ray crystallography to adopt an octahedral structure, the central nickel centre being coordinated by four water molecules and two acetate ligands. It may be dehydrated in vacuo, by reaction with acetic anhydride or by heat.
Safety
Nickel salts are toxic, carcinogenic and irritate the skin.
References
- M. A. Mohamed, S. A. Halawy, M. M. Ebrahim: "Non-isothermal decomposition of nickel acetate tetrahydrate", in: Journal of Analytical and Applied Pyrolysis, 1993, 27 (2), S. 109–110. doi:10.1016/0165-2370(93)80002-H.
- G. A. M. Hussein, A. K. H. Nohman, K. M. A. Attyia: "Characterization of the decomposition course of nickel acetate tetrahydrate in air", in: Journal of Thermal Analysis and Calorimetry, 1994, 42, S. 1155–1165; doi:10.1007/BF02546925.
- Downie, T. C.; Harrison, W.; Raper, E. S.; Hepworth, M. A. (15 March 1971). "A three-dimensional study of the crystal structure of nickel acetate tetrahydrate". Acta Crystallographica Section B. 27 (3): 706–712. Bibcode:1971AcCrB..27..706D. doi:10.1107/S0567740871002802.
- "Nickel metal and other compounds (as Ni)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Van Niekerk, J. N.; Schoening, F. R. L. (1953). "The crystal structures of nickel acetate, Ni(CH3COO)2·4H2O, and cobalt acetate, Co(CH3COO)2·4H2O". Acta Crystallogr. 6 (7): 609–612. doi:10.1107/S0365110X5300171X.
- Lascelles, Keith; Morgan, Lindsay G.; Nicholls, David; Beyersmann, Detmar (2005). "Nickel Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_235.pub2. ISBN 978-3527306732.
- Tappmeyer, W. P.; Davidson, Arthur W. (1963). "Cobalt and Nickel Acetates in Anhydrous Acetic Acid". Inorg. Chem. 2 (4): 823–825. doi:10.1021/ic50008a039.
| Nickel compounds | |
|---|---|
| Nickel(0) | |
| Nickel(II) | |
| Nickel(III) | |
| Nickel(IV) | |
| Acetyl halides and salts of the acetate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||