| Revision as of 00:10, 30 January 2015 editKeilana (talk | contribs)Autopatrolled, Administrators59,299 edits add data + CRUK ref← Previous edit | Revision as of 00:45, 30 January 2015 edit undoKeilana (talk | contribs)Autopatrolled, Administrators59,299 edits addNext edit → | ||
| Line 61: | Line 61: | ||
| === Protective factors === | === Protective factors === | ||
| Suppression of ovulation, which damages the ], and its associated ], is generally protective. This effect can be achieved by ], taking ]s, and ], all of which are protective factors.<ref name=Harrisons/> Each birth decreases risk of ovarian cancer more, and this effect is seen with up to five births. Combined oral contraceptives reduce the risk of ovarian cancer by up to 50%, and the protective effect of combined oral contraceptives can last 25 years after they are discontinued.<ref name=Hoffman35/> ] is protective because ]s are unable to reach the ovary and ] via the vagina, uterus, and Fallopian tubes.<ref name=Harrisons/> ] reduces the risk, and removal of both Fallopian tubes and ovaries (bilateral ]) dramatically reduces the risk of not only ovarian cancer, but breast cancer, as well.<ref name=Jayson/> A diet that includes large amounts of ], ], and ]s with low amounts of fat may be protective.<ref name=Hoffman35/> | Suppression of ovulation, which damages the ], and its associated ], is generally protective. This effect can be achieved by ], taking ]s, and ], all of which are protective factors.<ref name=Harrisons/> Each birth decreases risk of ovarian cancer more, and this effect is seen with up to five births. Combined oral contraceptives reduce the risk of ovarian cancer by up to 50%, and the protective effect of combined oral contraceptives can last 25 years after they are discontinued.<ref name=Hoffman35/> ] is protective because ]s are unable to reach the ovary and ] via the vagina, uterus, and Fallopian tubes.<ref name=Harrisons/> ] reduces the risk, and removal of both Fallopian tubes and ovaries (bilateral ]) dramatically reduces the risk of not only ovarian cancer, but breast cancer, as well.<ref name=Jayson/> A diet that includes large amounts of ], ], and ]s with low amounts of fat may be protective.<ref name=Hoffman35/> Specifically, a diet with non-starchy vegetables (e.g. ] and ]s) may help to reduce risk of ovarian cancer, but research is still ongoing in this area.<ref name=CRUKRisks/> | ||
| == Pathophysiology == | == Pathophysiology == | ||
Revision as of 00:45, 30 January 2015
Medical condition| Ovarian cancer | |
|---|---|
| Specialty | Oncology |
Ovarian cancer is a cancer that begins in an ovary. It results in abnormal cells that have the ability to invade or spread to other parts of the body. When this process begins, symptoms may be vague or not apparent, though they become more likely as the cancer progresses. Symptoms may include bloating, pelvic pain, and abdominal swelling, among others. Common areas where the cancer may spread include the lining of the abdomen, lymph nodes, lungs, and liver.
The risk of ovarian cancer is higher in people who ovulate more. Thus, those who have never had children are at increased risk, as are those who begin ovulation at a younger age or reach menopause at an older age. Other risk factors include hormone therapy after menopause, fertility medication, and obesity. Factors that decrease risk include hormonal birth control, tubal ligation, and breast feeding. About 10% of cases are related to inherited genetic risk, and those with the gene mutations BRCA1 or BRCA2 have about a 50% chance of developing the disease. The most common type of ovarian cancer, comprising more than 95% of cases, is ovarian carcinoma. Five main subtypes of ovarian carcinoma occur, of which high-grade serous is most common. These tumors are believed to start in the cells covering the ovaries, though some may form from the Fallopian tubes. Less common types include germ cell tumors and sex cord stromal tumors. The diagnosis is confirmed by examination of a biopsy usually removed during surgery.
Screening is not recommended in women who are at average risk, as evidence does not support a reduction in death and the high rate of false positive tests leads to unneeded surgery with its own risks. Those at very high risk may have their ovaries removed as a preventive measure. If caught and treated in an early stage, ovarian cancer may be curable. Treatments usually include some combination of surgery, radiation therapy, or chemotherapy. Outcomes depends on the extent of the disease and the subtype of cancer present. The overall five-year survival rate in the United States is 45%. Outcomes are worse in the developing world.
In 2012, ovarian cancer occurred in 239,000 women and resulted in 152,000 deaths worldwide. This made it the seventh-most common cancer and the eighth-most common cause of death from cancer in women. It is more common in North America and Europe than Africa and Asia.
Signs and symptoms
Signs and symptoms of ovarian cancer are frequently absent in early stages and when they exist, they may be subtle. In most cases, the symptoms persist for several months before being recognized and diagnosed, or they may be misdiagnosed as a condition such as irritable bowel syndrome. Unless ovarian torsion occurs because of the mass growing, the early stages of ovarian cancer tend to be painless. Most typical symptoms include bloating, abdominal or pelvic pain or discomfort, difficulty eating, fatigue, indigestion, heartburn, constipation, nausea, early satiety, and possibly urinary symptoms. If these symptoms start to occur more often or more severely than usual, especially after no significant history of those symptoms, the diagnosis should be considered. These symptoms are caused by a mass pressing on the other abdominopelvic organs or from metastases. In adolescents or children with ovarian tumors, the presenting symptoms can include severe abdominal pain, irritation of the peritoneum, or hemorrhage.As the cancer becomes more advanced, it can cause an accumulation of fluid in the abdomen. If the malignancy has not been diagnosed by the time it causes ascites, it is typically diagnosed shortly after.
Ovarian cancer symptoms can vary based on the subtype of cancer. Low malignant potential tumors, also known as borderline tumors, do not cause an increase in CA125 levels and are not identifiable with an ultrasound. The typical symptoms of a LMP tumor can include abdominal distension or pelvic pain. Particularly large masses tend to be benign or borderline.
Risk factors
Most of the risk factors for ovarian cancer are hormonal in nature. Not having children is a risk factor for ovarian cancer, likely because ovulation is not suppressed due to pregnancy. Both obesity and hormone replacement therapy raise the risk for ovarian cancer. Ovarian cancer is associated with increased age, family history of ovarian cancer (9.8-fold higher risk), anaemia (2.3-fold higher), abdominal pain (seven-fold higher), abdominal distension (23-fold higher), rectal bleeding (two-fold higher), postmenopausal bleeding (6.6-fold higher), appetite loss (5.2-fold higher), and weight loss (two-fold higher).
Hormones
Use of fertility medication may contribute to borderline ovarian tumor formation, but the link is disputed and difficult to study. Those who have been treated for infertility but remain nulliparous are at higher risk for epithelial ovarian cancer; however, those who are successfully treated for infertility and subsequently give birth are at no higher risk. This may be due to shedding of precancerous cells during pregnancy, but the cause remains unclear. Hormonal conditions such as polycystic ovary syndrome and endometriosis are associated with ovarian cancer, but the link is not completely confirmed. Postmenopausal hormone replacement therapy with estrogen likely increases the risk of ovarian cancer, but the association has not be confirmed in a large-scale study.
Long periods of continuous ovulation are thought to be the main non-genetic cause of epithelial ovarian cancer. This is because the cells are constantly stimulated to divide while ovulatory cycles continue. Therefore, people who have borne children are at twice the risk of ovarian cancer than people who have had children. A longer period of ovulation caused by early first menstruation or late menopause is also a risk factor.
Genetics
Further information: Hereditary breast-ovarian cancer syndrome
The major genetic risk factor for ovarian cancer is a mutation in BRCA1 or BRCA2, DNA mismatch repair genes. This occurs in 10% of ovarian cancer cases. Only mutation in one allele is needed to be at high risk for ovarian cancer, because the risky mutations are autosomal dominant. The gene can be inherited through either the maternal or paternal line, but has variable penetrance. Though mutations in these genes are usually associated with increased risk of breast cancer, they also carry a 30-50% lifetime risk of ovarian cancer, a risk that peaks in a woman's 40s-50s. This risk is also cited as 40-60% and 39-46%. Mutations in BRCA2 are less risky than those with BRCA1, with a lifetime risk of 20-40%. This risk is also cited as 12-20%. On average, BRCA-associated cancers develop 15 years before their sporadic counterparts, because people who inherit the mutations on one copy of their gene only need one mutation to start the process of carcinogenesis, whereas people with two normal genes would need to acquire two mutations.
In the United States, five of 100 women with a first-degree relative with ovarian cancer will eventually get ovarian cancer themselves, triple the risk of women with unaffected family members. Seven of 100 women with two or more relatives with ovarian cancer will eventually get ovarian cancer. In general, 5-10% of ovarian cancer cases have a genetic cause.
A strong family history of endometrial cancer, colon cancer, or other gastrointestinal cancers may indicate the presence of a syndrome known as hereditary nonpolyposis colorectal cancer (also known as Lynch syndrome), which confers a higher risk for developing ovarian cancer, among many other types of cancer. The risk of ovarian cancer for an individual with HNPCC is between 10 and 12 percent. Lynch syndrome is caused by mutations in mismatch repair genes, including MSH2, MLH1, MLH6, PMS1, and PMS2. People of Icelandic descent, European Jewish descent/Ashkenazi Jewish descent, and Hungarian descent are at higher risk for epithelial ovarian cancer.
Several rare genetic disorders are associated with specific subtypes of ovarian cancer. Peutz–Jeghers syndrome, a rare genetic disorder, also predisposes people to sex cord tumour with annular tubules. Ollier disease and Maffucci syndrome are associated with granulosa cell tumors in children and may be associated with Sertoli-Leydig tumors. Benign fibromas are associated with nevoid basal cell carcinoma syndrome.
Environmental factors
Industrialized nations, with the exception of Japan, have high rates of epithelial ovarian cancer, which may be due to diet in those countries. White people are at a 30-40% higher risk for ovarian cancer when compared to Black and Hispanic people, likely due to socioeconomic factors: White women have fewer children and different rates of gynecologic surgeries that affect risk for ovarian cancer.
Other
Alcohol consumption does not appear to be related to ovarian cancer. Other factors that have been investigated, such as talc use on the perineum, smoking, and infection with human papilloma virus (the cause of some cases of cervical cancer) have been disproven as risk factors for ovarian cancer. However, the carcinogenicity of perineal talc is controversial, because it can act as an irritant if it travels through the reproductive tract to the ovaries.
Increased age (up to the 70s) is a risk factor for epithelial ovarian cancer because more mutations in cells can accumulate and eventually cause cancer. Women over 80 are at slightly lower risk.
Protective factors
Suppression of ovulation, which damages the ovarian epithelium, and its associated inflammation, is generally protective. This effect can be achieved by having children, taking combined oral contraceptives, and breast feeding, all of which are protective factors. Each birth decreases risk of ovarian cancer more, and this effect is seen with up to five births. Combined oral contraceptives reduce the risk of ovarian cancer by up to 50%, and the protective effect of combined oral contraceptives can last 25 years after they are discontinued. Tubal ligation is protective because carcinogens are unable to reach the ovary and fimbriae via the vagina, uterus, and Fallopian tubes. Hysterectomy reduces the risk, and removal of both Fallopian tubes and ovaries (bilateral salpingo-oophorectomy) dramatically reduces the risk of not only ovarian cancer, but breast cancer, as well. A diet that includes large amounts of carotene, fiber, and vitamins with low amounts of fat may be protective. Specifically, a diet with non-starchy vegetables (e.g. broccoli and onions) may help to reduce risk of ovarian cancer, but research is still ongoing in this area.
Pathophysiology
| Gene mutated | Mutation type | Subtype | Prevalence |
|---|---|---|---|
| AKT1 | amplification | 3% | |
| AKT2 | amplification/mutation | 6%, 20% | |
| ARID1A | point mutation | endometrioid and clear cell | |
| BRAF | point mutation | low-grade serous | 0.5% |
| BRCA1 | nonsense mutation | high-grade serous | 5% |
| BRCA2 | frameshift mutation | high-grade serous | 3% |
| CCND1 | amplification | 4% | |
| CCND2 | upregulation | 15% | |
| CCNE1 | amplification | 20% | |
| CDK12 | high-grade serous | ||
| CDKN2A | downregulation (30%) and deletion (2%) | 32% | |
| CTNNB1 | clear cell | ||
| DYNLRB1 (km23) | mutation | 42% | |
| EGFR | amplification/overexpression | 20% | |
| ERBB2 (Her2/neu) | amplification/overexpression | mucinous and low-grade serous | 30% |
| FMS | coexpression with CSF-1 | 50% | |
| JAG1 | amplification | 2% | |
| JAG2 | amplification | 3% | |
| KRAS | amplification | mucinous and low-grade serous | 11% |
| MAML1 | amplification and point mutation | 2% | |
| MAML2 | amplification and point mutation | 4% | |
| MAML3 | amplification | 2% | |
| MLH1 | 1% | ||
| NF1 | deletion (8%) and point mutation (4%) | high-grade serous | 12% |
| NOTCH3 | amplification and point mutation | 11% | |
| NRAS | low-grade serous | ||
| PIK3C3 (PI3K3) | amplification/mutation | 12-20% | |
| PIK3CA | amplification | endometrioid and clear cell | 18% |
| PPP2R1A | endometrioid and clear cell | ||
| PTEN | deletion | endometrioid and clear cell | 7% |
| RB1 | deletion (8%) and point mutation (2%) | 10% | |
| TGF-β | mutation/overexpression | 12% | |
| TP53 | mutation/overexpression | high-grade serous | 20-50% |
| TβRI | mutation | 33% | |
| TβRII | mutation | 25% |
Ovarian cancer forms when errors in normal ovarian cell growth occur. Usually, when cells grow old or get damaged, they die, and new cells take their place. Cancer starts when new cells form unneeded, and old or damaged cells do not die as they should. The buildup of extra cells often forms a mass of tissue called a growth or tumor. These abnormal cancer cells have many genetic abnormalities that cause them to grow excessively. Continuous ovulation for a long time means more repair of the ovary by dividing cells, which can acquire mutations.
Overall, the most common gene mutations in ovarian cancer occur in NF1, BRCA1, BRCA2, and CDK12. Type-I ovarian cancers tend to have microsatellite instability in several genes, including BRAF, KRAS, and PTEN, which are tumor suppressor genes. Type-II cancers have different genes mutated, including p53, BRCA1, and BRCA2. Low-grade cancers tend to have mutations in KRAS, whereas cancers of any grade that develop from low malignant potential tumors tend to have mutations in p53. Serous cancers that have BRCA mutations also inevitably have p53 mutations, indicating that the removal of both functional genes is important for cancer to develop.
In 50% of high-grade serous cancers, homologous recombination DNA repair is dysfunctional, as are the notch and FOXM1 signaling pathways. They also almost always have p53 mutations. Beyond this, mutations in high-grade serous carcinoma are hard to characterize beyond their high degree of genomic instability. BRCA1 and BRCA2 are essential for homologous recombination DNA repair, and germline mutations in these genes are found in about 15% of people with ovarian cancer. The most common mutations in BRCA1 and BRCA2 are the frameshift mutations that originated in a small founding population of Ashkenazi Jews.
Almost 100% of the rare mucinous carcinomas have mutations in KRAS and amplifications of ERBB2 (also known as Her2/neu). Overall, 20% of ovarian cancers have mutations in Her2/neu.
Serous carcinomas may develop from serous tubal intraepithelial carcinoma, rather than developing spontaneously from ovarian tissue. Other carcinomas develop from cortical inclusion cysts, which are groups of epithelial ovarian cells inside the stroma.
Diagnosis
Examination

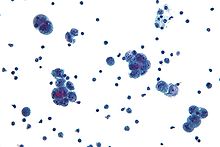
Diagnosis of ovarian cancer starts with a physical examination (including a pelvic examination), a blood test (for CA-125 and sometimes other markers), and transvaginal ultrasound. Sometimes a rectovaginal examination is used to help plan a surgery. The diagnosis must be confirmed with surgery to inspect the abdominal cavity, take biopsies (tissue samples for microscopic analysis), and look for cancer cells in the abdominal fluid. This helps to determine if an ovarian mass is benign or malignant.
Ovarian cancer at its early stages (I/II) is difficult to diagnose until it spreads and advances to later stages (III/IV) s because most symptoms are nonspecific and thus of little use in diagnosis. The serum BHCG level should be measured in any female in whom pregnancy is a possibility. In addition, serum alpha-fetoprotein and lactate dehydrogenase should be measured in young girls and adolescents with suspected ovarian tumors because the younger the patient, the greater the likelihood of a malignant germ cell tumor.
When an ovarian malignancy is included in the list of diagnostic possibilities, a limited number of laboratory tests are indicated. A complete blood count and serum electrolyte test should be obtained in all patients. When an ovarian cancer is present, these tests often show a high number of platelets (20-25% of people) and low blood sodium levels due to chemical signals secreted by the tumor. A blood test for a marker molecule called CA-125 is useful in differential diagnosis and in follow up of the disease, but it by itself has not been shown to be an effective method to screen for early-stage ovarian cancer due to its unacceptable low sensitivity and specificity. Another test used is OVA1. CA-125 levels are not accurate in early stage ovarian cancer, as fully half of stage I ovarian cancer patients have a normal CA-125 level.
Current research is looking at ways to combine tumor marker proteomics along with other indicators of disease (i.e. radiology and/or symptoms) to improve accuracy. The challenge in such an approach is that the disparate prevalence of ovarian cancer means that even testing with very high sensitivity and specificity will still lead to a number of false positive results (i.e. performing surgical procedures in which cancer is not found intraoperatively). However, the contributions of proteomics are still in the early stages and require further refining. Current studies on proteomics mark the beginning of a paradigm shift towards individually tailored therapy.
A physical examination, including a pelvic examination, and a pelvic ultrasound (which can be a transvaginal ultrasound) are essential for diagnosis. Physical examination may reveal increased abdominal girth and/or ascites (fluid within the abdominal cavity). Pelvic examination may reveal an ovarian or abdominal mass. CT scanning is preferred to assess the extent of the tumor in the abdominopelvic cavity, though magnetic resonance imaging can also be used. CT scanning can also be useful for finding omental caking or differentiating fluid from solid tumor in the abdomen, especially in low malignant potential tumors.
To definitively diagnose ovarian cancer, a surgical procedure to inspect the abdomen is required. This can be an open procedure (laparotomy, incision through the abdominal wall) or keyhole surgery (laparoscopy). During this procedure, suspicious areas are removed and sent for microscopic analysis. Usually, this includes a unilateral salpingo-oophorectomy, removal of just the affected ovary and Fallopian tube. Fluid from the abdominal cavity can also be analyzed for cancerous cells. If cancer is found, this procedure can also determine its spread (which is a form of tumor staging).
Risk scoring
A widely recognized method of estimating the risk of malignant ovarian cancer based on initial workup is the risk of malignancy index (RMI). Generally, an RMI score of over 200 is considered to indicate high risk for ovarian cancer.
The RMI is calculated as:
- RMI = ultrasound score x menopausal score x CA-125 level in U/ml.
Two methods are used to determine the ultrasound score and menopausal score, with the resultant RMI being called RMI 1 and RMI 2, respectively, depending on what method is used.
| Feature | RMI 1 | RMI 2 |
|---|---|---|
|
Ultrasound abnormalities:
|
|
|
| Menopausal score |
|
|
| CA-125 | Quantity in U/ml | Quantity in U/ml |
Another method for quantitating risk of ovarian cancer is the Risk of Ovarian Cancer Algorithm (ROCA), which graphs CA-125 measurements over time and determines if it is increasing at a rate high enough to warrant transvaginal ultrasound.
Pathology

Ovarian cancers are classified according to the microscopic appearance of their structures (histology or histopathology). Histology dictates many aspects of clinical treatment, management, and prognosis. According to SEER, the types of ovarian cancers in women age 20and over are:
| Percent of ovarian cancers in women age 20+ |
Histology | Five-year RSR |
|---|---|---|
| 89.7 | Surface epithelial-stromal tumor (adenocarcinoma) | 54.4 |
| 26.4 | Papillary serous cystadenocarcinoma | 21.0 |
| 15.9 | "Borderline" adenocarcinoma (underestimated - short data collection interval) |
98.2 |
| 12.6 | Adenocarcinoma, not otherwise specified | 18.3 |
| 9.8 | Endometrioid tumor | 70.9 |
| 5.8 | Serous cystadenocarcinoma | 44.2 |
| 5.5 | Papillary | 21.0 |
| 4.2 | Mucinous cystadenocarcinoma | 77.7 |
| 4.0 | Clear-cell ovarian tumor | 61.5 |
| 3.4 | Mucinous adenocarcinoma | 49.1 |
| 1.3 | Cystadenocarcinoma | 50.7 |
| 5.5 | Carcinoma | |
| 4.1 | Carcinoma not otherwise specified | 26.8 |
| 1.1 | Sex cord-stromal tumour | 87.8 |
| 0.3 | Other carcinomas, specified | 37.3 |
| 1.7 | Mullerian tumor | 29.8 |
| 1.5 | Germ cell tumor | 91.0 |
| 0.8 | Teratoma | 89.1 |
| 0.5 | Dysgerminoma | 96.8 |
| 0.3 | Other, specified | 85.1 |
| 0.6 | Not otherwise specified | 23.0 |
| 0.5 | Epidermoid (squamous cell carcinoma) | 51.3 |
| 0.2 | Brenner tumor | 67.9 |
| 0.2 | Other, specified | 71.7 |
Ovarian cancers are histologically and genetically divided into type I or type II. Type I cancers are of low histological grade, and include endometrioid, mucinous, and clear-cell carcinomas. Type II cancers are of higher histological grade and include serous carcinoma and carcinosarcoma.
Epithelial carcinoma

Surface epithelial-stromal tumour, also known as ovarian epithelial carcinoma, is the most common type of ovarian cancer. It includes serous tumour, endometrioid tumor, and mucinous cystadenocarcinoma. Less common tumors are malignant Brenner tumor and transitional cell carcinoma of the ovary.
Serous carcinoma
Most people with epithelial ovarian carcinoma have a high-grade serous carcinoma. Low-grade serous carcinoma is less aggressive than high-grade serous carcinomas, though it does not typically respond well to chemotherapy or hormonal treatments.
Clear-cell carcinoma
Clear-cell ovarian carcinomas do not typically respond well to chemotherapy and may be related to endometriosis.
Sex cord stromal tumor
Sex cord-stromal tumor, including estrogen-producing granulosa cell tumor, the benign thecoma, and virilizing Sertoli-Leydig cell tumor or arrhenoblastoma, accounts for 7% of ovarian cancers. They occur most frequently in women between 50 and 69 years of age, but can occur in women of any age, including young girls. They are not typically aggressive and are usually unilateral.
Several different cells from the mesenchyme can give rise to sex-cord or stromal tumors. These include fibroblasts and endocrine cells. The symptoms of a sex-cord or stromal ovarian tumor can differ from other types of ovarian cancer. Common signs and symptoms include ovarian torsion, hemorrhage from or rupture of the tumor, an abdominal mass, and hormonal disruption. In children, isosexual precocious pseudopuberty may occur with granulosa cell tumors since they produce estrogen. These tumors cause abnormalities in menstruation (excessive bleeding, infrequent menstruation, or no menstruation) or postmenopausal bleeding. Because these tumors produce estrogen, they can cause or occur at the same time as endometrial cancer or breast cancer. Other sex-cord/stromal tumors present with distinct symptoms. Sertoli-Leydig cell tumors cause virilization and excessive hair growth due to the production of testosterone and androstenedione, which can also cause Cushing's syndrome in rare cases. Also, sex-cord stromal tumors occur that do not cause a hormonal imbalance, including benign fibromas, which cause ascites and hydrothorax.
Germ cell tumor
Germ cell tumor accounts for about 30% of ovarian tumors, but only 5% of ovarian cancers, because most germ-cell tumors are teratomas and most teratomas are benign. Malignant teratomas tend to occur in older women, when one of the germ layers in the tumor develops into a squamous cell carcinoma. Germ-cell tumors tend to occur in young women (20s-30s) and girls, making up 70% of the ovarian cancer seen in that age group. Germ-cell tumors can include dysgerminomas, teratomas, yolk sac tumors/endodermal sinus tumors, and choriocarcinomas, when they arise in the ovary. Some germ-cell tumors have an isochromosome 12, where one arm of chromosome 12 is deleted and replaced with a duplicate of the other. While the overall prognosis of germ-cell tumors is favorable, it can vary substantially with specific histology; for instance, the prognosis of the most common germ cell tumor (dysgerminomas) tends to be good, whilst the second-most common (endodermal sinus tumor) tends to have a poor prognosis. Overall, they metastasize more frequently than epithelial ovarian cancers. In addition, the cancer markers used vary with tumor type: choriocarcinomas are monitored with beta-HCG and endodermal sinus tumors with alpha-fetoprotein.
Germ-cell tumors are typically discovered when they become large, palpable masses. However, like sex cord tumors, they can cause ovarian torsion or hemorrhage and, in children, isosexual precocious puberty. They frequently metastasize to nearby lymph nodes, especially para-aortic and pelvic lymph nodes.
Dysgerminoma
Dysgerminoma accounts for 35% of ovarian cancer in young women. These tumors may have mutations in the KIT gene, a mutation known for its role in gastrointestinal stromal tumor. People with an XY karyotype and ovaries (gonadal dysgenesis) who develop a unilateral dysgerminoma are at risk for a gonadoblastoma in the other ovary, and in this case, both ovaries are usually removed when a unilateral dysgerminoma is discovered. However, in general, dysgerminomas are bilateral 10-15% of the time.
Mixed tumors
Mixed tumors contain elements of more than one of the above classes of tumor histology.
Secondary ovarian cancer
Ovarian cancer can also be a secondary cancer, the result of metastasis from a primary cancer elsewhere in the body. About 7% of ovarian cancers are due to metastases, while the rest are primary cancers. Common primary cancers are breast cancer, colon cancer, appendiceal cancer, and stomach cancer (primary gastric cancers that metastasize to the ovary are called Krukenberg tumors). Surface epithelial-stromal tumor can originate in the peritoneum (the lining of the abdominal cavity), in which case the ovarian cancer is secondary to primary peritoneal cancer, but treatment is basically the same as for primary surface epithelial-stromal tumor involving the peritoneum.
Low malignant potential tumors
Low malignant potential ovarian tumors, also called borderline tumors, have some benign and some malignant features. They develop earlier than epithelial ovarian cancer, around the age of 40-49. They typically do not have extensive invasion; 10% of LMP tumors have areas of stromal microinvasion (<3mm, <5% of tumor). LMP tumors have other abnormal features, including increased mitosis, changes in cell size or nucleus size, abnormal nuclei, cell stratification, and small projections on cells (papillary projections). Serous and/or mucinous characteristics can be seen on histological examination, and serous histology makes up the overwhelming majority of advanced LMP tumors. More than 80% of LMP tumors are Stage I; 15% are stage II and III and less than 5% are stage IV.
Staging
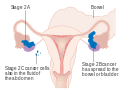
Ovarian cancer is staged using the FIGO staging system and uses information obtained after surgery, which can include a total abdominal hysterectomy, removal of (usually) both ovaries and Fallopian tubes, (usually) the omentum, pelvic (peritoneal) washings, and pelvic biopsies for cytopathology. Around 30% of ovarian cancers that appear confined to the ovary have metastasized microscopically, which is why even stage-I cancers must be staged completely. The AJCC stage is the same as the FIGO stage. The AJCC staging system describes the extent of the primary tumor (T), the absence or presence of metastasis to nearby lymph nodes (N), and the absence or presence of distant metastasis (M). The most common stage at diagnosis is stage IIIc, with over 70% of diagnoses.

The FIGO stages are as follows:
| Stage | Description | |||
|---|---|---|---|---|
| I | Cancer is completely limited to the ovary | |||
| IA | involves one ovary, capsule intact, no tumor on ovarian surface, negative washings | |||
| IB | involves both ovaries; capsule intact; no tumor on ovarian surface; negative washings | |||
| IC | tumor involves one or both ovaries | |||
| IC1 | surgical spill | |||
| IC2 | capsule has ruptured or tumor on ovarian surface | |||
| IC3 | positive ascites or washings | |||
| II | pelvic extension of the tumor (must be confined to the pelvis) or primary peritoneal tumor, involves one or both ovaries | |||
| IIA | tumor found on uterus or fallopian tubes | |||
| IIB | tumor elsewhere in the pelvis | |||
| III | cancer found outside the pelvis or in the retroperitoneal lymph nodes, involves one or both ovaries | |||
| IIIA | metastasis in retroperitoneal lymph nodes or microscopic extrapelvic metastasis | |||
| IIIA1 | metastasis in retroperitoneal lymph nodes | |||
| IIIA1(i) | the metastasis is less than 10 mm in diameter | |||
| IIIA1(ii) | the metastasis is greater than 10 mm in diameter | |||
| IIIA2 | microscopic metastasis in the peritoneum, regardless of retroperitoneal lymph node status | |||
| IIIB | metastasis in the peritoneum less than or equal to 2 cm in diameter, regardless of retroperitoneal lymph node status; or metastasis to liver or spleen capsule | |||
| IIIC | metastasis in the peritoneum greater than 2 cm in diameter, regardless of retroperitoneal lymph node status; or metastasis to liver or spleen capsule | |||
| IV | distant metastasis (i.e. outside of the peritoneum) | |||
| IVA | pleural effusion containing cancer cells | |||
| IVB | metastasis to distant organs (including the parenchyma of the spleen or liver), or metastasis to the inguinal and extra-abdominal lymph nodes |
Para-aortic lymph node metastases are considered regional lymph nodes (Stage IIIC). As there is only one para-aortic lymph node intervening before the thoracic duct on the right side of the body, the ovarian cancer can rapidly spread to distant sites such as the lung.
The AJCC/TNM staging system includes three categories for ovarian cancer, T, N, and M. The T category contains three other subcategories, T1, T2, and T3, each of them being classified according to the place where the tumor has developed (in one or both ovaries, inside or outside the ovary). The T1 category of ovarian cancer describes ovarian tumors that are confined to the ovaries, and which may affect one or both of them. The subsubcategory T1a is used to stage cancer found in only one ovary, which has left the capsule intact and which cannot be found in the fluid taken from the pelvis. Cancer that has not affected the capsule is confined to the inside of the ovaries and cannot be found in the fluid taken from the pelvis, but has affected both ovaries, is staged as T1b. T1c category describes a type of tumor that can affect one or both ovaries, and which has grown through the capsule of an ovary or it is present in the fluid taken from the pelvis. T2 is a more advanced stage of cancer. In this case, the tumor has grown in one or both ovaries and is spread to the uterus, Fallopian tubes, or other pelvic tissues. Stage T2a is used to describe a cancerous tumor that has spread to the uterus or the Fallopian tubes (or both) but which is not present in the fluid taken from the pelvis. Stages T2b and T2c indicate cancer that metastasized to other pelvic tissues than the uterus and Fallopian tubes and which cannot be seen in the fluid taken from the pelvis, respectively tumors that spread to any of the pelvic tissues (including uterus and Fallopian tubes), but which can also be found in the fluid taken from the pelvis. T3 is the stage used to describe cancer that has spread to the peritoneum. This stage provides information on the size of the metastatic tumors (tumors that are located in other areas of the body, but are caused by ovarian cancer). These tumors can be very small, visible only under the microscope (T3a), visible but not larger than 2 cm (T3b) and bigger than 2 cm (T3c).
This staging system also uses N categories to describe cancers that have or not spread to nearby lymph nodes. The two N categories are N0, which indicates the cancerous tumors have not affected the lymph nodes, and N1, which indicates the involvement of lymph nodes close to the tumor.
The M categories in the AJCC/TNM staging system provide information on whether the ovarian cancer has metastasized to distant organs such as liver or lungs. M0 indicates the cancer did not spread to distant organs and M1 category is used for cancer that has spread to other organs of the body.
The AJCC/TNM staging system also contains a Tx and a Nx subcategory which indicates the extent of the tumor cannot be described because of insufficient data, respectively the involvement of the lymph nodes cannot be described because of the same reason.
The ovarian cancer stages are made up by combining the TNM categories in the following manner:
- Stage I: T1+N0+M0
- IA: T1a+N0+M0
- IB: T1b+N0+M0
- IC: T1c+N0+M0
- Stage II: T2+N0+M0
- IIa: T2a+N0+M0
- IIB: T2b+N0+M0
- IIC: T2c+N0+M0
- Stage III: T3+ N0+M0
- IIIA: T3a+ N0+M0
- IIIB: T3b+ N0+M0
- IIIC: T3c+ N0+M0 or Any T+N1+M0
- Stage IV: Any T+ Any N+M1
In addition to being staged, like all cancers, ovarian cancer is also graded. The histologic grade of a tumor measures how abnormal or malignant its cells look under the microscope. The four grades indicate the likelihood of the cancer to spread and the higher the grade, the more likely for this to occur. Grade 0 is used to describe noninvasive tumors. Grade 0 cancers are also referred to as borderline tumors. Grade 1 tumors have well differentiated cells (look very similar to the normal tissue) and are the ones with the best prognosis. Grade 2 tumors are also called moderately well-differentiated and they are made up of cells that resemble the normal tissue. Grade 3 tumors have the worst prognosis and their cells are abnormal, referred to as poorly differentiated.
Screening
The only screening recommended for all women is an annual pelvic examination. This is not very effective in detecting early ovarian cancer because it is usually only palpable in advanced stages. Ovarian cancer screening is of high clinical interest because the disease is not typically detectable at its early stages, when it is the most curable. Screening is not recommended using CA-125 measurements, HE4 levels, ultrasound, or adnexal palpation in women who are at average risk. Screening for any type of cancer must be accurate and reliable—it needs to accurately detect the disease and it must not give false positive results in people who do not have cancer.
Ovarian cancer has low prevalence, even in the high-risk group of women from the ages of 50 to 60 (about one in 2000), and screening of women with average risk is more likely to give ambiguous results than detect a problem which requires treatment. Because ambiguous results are more likely than detection of a treatable problem, and because the usual response to ambiguous results is invasive interventions, in women of average risk, the potential harms of having screening without an indication outweigh the potential benefits. The purpose of screening is to diagnose ovarian cancer at an early stage, when it is more likely to be treated successfully. Screening with transvaginal ultrasound, pelvic examination, and CA-125 levels can be used instead of prophylactic surgery in women who have BRCA1 or BRCA2 mutations. This strategy has shown some success.
Prevention
People with strong genetic risk for ovarian cancer may consider the surgical removal of their ovaries as a preventative measure. This is often done after completion of childbearing years. This reduces the chances of developing both breast cancer (by around 50%) and ovarian cancer (by about 96%) in people at high risk. Women with BRCA gene mutations usually also have their Fallopian tubes removed at the same time (salpingo-oophorectomy), since they also have an increased risk of Fallopian tube cancer. However, these statistics may overestimate the risk reduction because of how they have been studied.
People with a significant family history for ovarian cancer are often referred to a genetic counselor to see if they should be tested for BRCA mutations.
Management
Treatment usually involves chemotherapy and surgery, and sometimes radiotherapy. Surgical treatment may be sufficient for well-differentiated malignant tumors and confined to the ovary. Addition of chemotherapy may be required for more aggressive tumors confined to the ovary. For patients with advanced disease, a combination of surgical reduction with a combination chemotherapy regimen is standard. Borderline tumors, even following spread outside of the ovary, are managed well with surgery, and chemotherapy is not seen as useful.
Surgery
Surgery is the preferred treatment and is frequently necessary to obtain a tissue specimen for differential diagnosis via its histology. The type of surgery depends upon how widespread the cancer is when diagnosed (the cancer stage), as well as the presumed type and grade of cancer. The surgeon, who is usually a specialized gynecologic oncology surgeon, may remove one (unilateral oophorectomy) or both ovaries (bilateral oophorectomy), the Fallopian tubes (salpingectomy), the uterus (hysterectomy), and the omentum (omentectomy). Typically, all of these are removed. For low-grade, unilateral stage-IA cancers, only the involved ovary (which must be unruptured) and Fallopian tube will be removed. This can be done especially in young females who wish to preserve their fertility. However, a risk of microscopic metastases exists and staging must be completed. If a tumor in a premenopausal woman is determined to be a low malignant potential tumor during surgery, and it is clearly stage I cancer, only the affected ovary is removed. For postmenopausal women with low malignant potential tumors, hysterectomy with bilateral salpingo-oophorectomy is still the preferred option. During staging, the appendix should be examined or removed. This is particularly important with mucinous tumors. In children or adolescents with ovarian cancer, surgeons typically attempt to preserve one ovary to allow for the completion of puberty, but if the cancer has spread, this is not always possible. Dysgerminomas in particular tend to affect both ovaries: 8-15% of dysgerminomas are present in both ovaries. People with low-grade (well-differentiated) tumors are typically treated only with surgery.
In advanced cancers, where complete removal is not an option, as much tumor as possible is removed in a procedure called debulking surgery. This surgery is not always successful, and is less likely to be successful in women with extensive metastases in the peritoneum, stage- IV disease, cancer in the transverse fissure of the liver, mesentery, or diaphragm, and large areas of ascites. Debulking surgery is usually only done once. More complete debulking is associated with better outcomes: women with no macroscopic evidence of disease after debulking have a median survival of 39 months, as opposed to 17 months with less complete surgery.
To fully stage ovarian cancer, lymphadenectomy should be included in the surgery, but a significant survival benefit to this practice may not happen. This is particularly important in germ cell tumors because they frequently metastasize to nearby lymph nodes.
If ovarian cancer recurs, secondary surgery is sometimes a treatment option. This depends on how easily the tumor can be removed, how much fluid has accumulated in the abdomen, and overall health.
The major side effect of an oophorectomy in younger women is early menopause, which can cause osteoporosis. After surgery, hormone replacement therapy can be considered, especially in younger women. This therapy can consist of a combination of estrogen and progesterone, or estrogen alone. Estrogen alone is safe after hysterectomy; when the uterus is still present, unopposed estrogen dramatically raises the risk of endometrial cancer.
Chemotherapy
Chemotherapy has been a general standard of care for ovarian cancer for decades, although with highly variable protocols. Chemotherapy is used after surgery to treat any residual disease, if appropriate. In some cases, there may be reason to perform chemotherapy first, followed by surgery. This is called "neoadjuvant chemotherapy", and is common when a tumor cannot be completely removed or optimally debulked via surgery. If a unilateral salpingo-oophorectomy or other surgery is performed, additional chemotherapy, called "adjuvant chemotherapy", can be given. Chemotherapies used in ovarian cancer include paclitaxel, cisplatin, topotecan, and gemcitabine. Germ-cell malignancies are treated differently - a regimen of bleomycin, etoposide, and cisplatin (BEP chemotherapy) is used.
Chemotherapy in ovarian cancer typically consists of platins, a group of platinum-based drugs. Carboplatin is given in combination with either paclitaxel or docetaxel; the typical combination is carboplatin with paclitaxel. Three-drug regimens have not been found to be more effective. Chemotherapy can be given intravenously or in the peritoneal cavity.
If ovarian cancer recurs, it is considered partially platinum-sensitive or platinum-resistant, based on the time since the last recurrence treated with platins: partially platinum-sensitive cancers recurred 6–12 months after last treatment, and platinum-resistant cancers have an interval of less than 6 months. Second-line chemotherapy should be given only after the cancer becomes symptomatic, because no difference in survival is seen between treating asymptomatic (elevated CA-125) and symptomatic recurrences. For platinum-sensitive tumors, platins are the drugs of choice for second-line chemotherapy, in combination with other cytotoxic agents. Regimens include carboplatin combined with pegylated liposomal doxorubicin, gemcitabine, or paclitaxel. For platinum-resistant tumors, there are no high-efficacy chemotherapy options. Single-drug regimens (doxorubicin or topotecan) do not have high response rates, but topotecan is used in some cases.
In people with BRCA mutations, platinum chemotherapy is more effective. Olaparib, a PARP inhibitor, was approved by the U.S. Food and Drug Administration for use in BRCA-associated ovarian cancer that had previously been treated with chemotherapy. Germ-cell tumors and malignant sex-cord/stromal tumors are treated with chemotherapy, though dysgerminomas and sex-cord tumors are not typically very responsive.
Radiation therapy
Dysgerminomas are most effectively treated with radiation, though this can cause infertility and is being phased out in favor of chemotherapy.
Radiation therapy is not effective for advanced stages because when vital organs are in the radiation field, a high dose cannot be safely delivered. Radiation therapy is then commonly avoided in such stages as the vital organs may not be able to withstand the problems associated with these ovarian cancer treatments. Radiation therapy does not improve survival in people with well-differentiated tumors.
Hormonal therapy
Despite the fact that 60% of ovarian tumors have estrogen receptors, ovarian cancer is only rarely responsive to hormonal treatments. Estrogen alone does not have an effect on the cancer, and tamoxifen and letrozole are rarely effective.
Follow-up
Specific follow-up depends on, for example, the type and stage of ovarian cancer, the treatment, and the presence of any symptoms. Usually, a check-up appointment is made about every 2 to 3 months initially, followed by twice per year for up to 5 years. For epithelial ovarian cancers, the most common test upon follow-up is CA-125 level. However, treatment based only on elevated CA-125 levels and not any symptoms can increase side effects without any prolongation of life, so the implication of the outcome of a CA-125 test should be discussed before taking it. The recommendation as of 2014 is recurrent cancer may be present if the CA-125 level is twice normal.
For women with germ-cell tumors, follow-up tests generally include alpha-fetoprotein (AFP) and/or human chorionic gonadotropin. For women with stromal cancers, tests for hormones like estrogen, testosterone, and inhibin are sometimes helpful. Inhibin can also be useful for monitoring the progress of sex-cord tumors, along with mullerian inhibiting substance. AFP can also be used to monitor Sertoli-Leydig tumors.
Women with ovarian cancer should not have routine surveillance imaging to monitor the cancer unless new symptoms appear or tumor markers begin rising. Imaging without these indications is discouraged because it is unlikely to detect a recurrence, improve survival, and because it has its own costs and side effects. However, CT imaging can be used if desired, though this is not common.
Palliative care
Palliative care is a holistic treatment with a focus on relieving symptoms and increasing or maintaining quality of life. It has been recommended as part of the treatment plan for any person with advanced ovarian cancer or patients with significant symptoms.
Palliative care can entail treatment of symptoms and complications of the cancer, including pain, nausea, constipation, ascites, bowel obstruction, edema, and mucositis. Especially if the cancer advances and becomes incurable, treatment of symptoms becomes one of the main goals of therapy. Palliative care can also entail helping with decision-making such as if or when hospice care is appropriate, and the preferred place for the patient at end of life care. Bowel obstruction can be treated with palliative surgery. Other treatments of complications can include total parenteral nutrition, a low-residue diet, and adequate pain control.
Prognosis

Ovarian cancer usually has a relatively poor prognosis. It is disproportionately deadly because it lacks any clear early detection or screening test, meaning most cases are not diagnosed until they have reached advanced stages. However, in some cases, ovarian cancer recurrences are chronically treatable.
Ovarian cancer metastasizes early in its development, often before it has been diagnosed. High-grade tumors metastasize more readily than low-grade tumors. Typically, tumor cells begin to metastasize by growing in the peritoneal cavity. More than 60% of women presenting with ovarian cancer have stage-III or stage-IV cancer, when it has already spread beyond the ovaries. Ovarian cancers shed cells into the naturally occurring fluid within the abdominal cavity. These cells can then implant on other abdominal (peritoneal) structures, included the uterus, urinary bladder, bowel, lining of the bowel wall, and omentum, forming new tumor growths before cancer is even suspected.
The five-year survival rate for all stages of ovarian cancer is 47%. For cases where a diagnosis is made early in the disease, when the cancer is still confined to the primary site, the five-year survival rate is 92.7%. While the overall five-year survival rate for all cancers combined has improved significantly: 68% for the general population diagnosed in 2001 (compared to 50% in the 1970s), ovarian cancer has a poorer outcome with a 47% survival rate (compared to 38% in the late 1970s). About 70% of women with advanced disease respond to initial treatment, most of whom attain complete remission, but half of these women experience a recurrence 1-4 years after treatment.
Ovarian cancer survival varies significantly with subtype. Dysgerminomas have a very favorable prognosis. In early stages, they have a five-year survival rate of 96.9%. Stage-III dysgerminomas have a five-year survival of 61%; when treated with BEP chemotherapy after incomplete surgical removal, dysgerminomas have a 95% two-year survival rate. Sex-cord/stromal malignancies also have a favorable prognosis; because they are slow-growing, even those with metastatic disease can survive a decade or more. Low malignant potential tumors usually only have a bad prognosis when there are invasive tumor implants found in the peritoneal cavity.
Complications of ovarian cancer can include spread of the cancer to other organs, progressive function loss of various organs, ascites, and intestinal obstructions, which can be fatal. Intestinal obstructions in multiple sites are the most common proximate cause of death. Intestinal obstruction in ovarian cancer can either be a true obstruction, where tumor blocks the intestinal lumen, or a pseudo-obstruction, when tumor prevents normal peristalsis. Continuous accumulation of ascites can be treated by placing a drain that can be self-drained.
Survival rates
Overall five-year survival rates for all types of ovarian cancer are presented below by stage and histologic grade:
| Stage | Survival |
|---|---|
| I | 90-95% |
| II | 70-80% |
| III | 20-50% |
| IV | 1-5% |
| Histologic grade | Survival |
|---|---|
| Low grade | 88% |
| Intermediate grade | 58% |
| High grade | 27% |
The survival rates given below are for the different types of ovarian cancer, according to American Cancer Society. They come from the National Cancer Institute, SEER, and are based on patients diagnosed from 2004 to 2010.
| Invasive epithelial ovarian cancer | |
|---|---|
| Stage | Relative five-year survival rate |
| I | 90% |
| IA | 94% |
| IB | 92% |
| IC | 85% |
| II | 70% |
| IIA | 78% |
| IIB | 73% |
| III | 39% |
| IIIA | 59% |
| IIIB | 52% |
| IIIC | 39% |
| IV | 17% |
| Ovarian stromal tumors | |
|---|---|
| Stage | Relative five-year survival rate |
| I | 95% |
| II | 78% |
| III | 65% |
| IV | 35% |
| Germ cell tumors of the ovary | |
|---|---|
| Stage | Relative 5-yr Survival Rate |
| I | 98% |
| II | 94% |
| III | 87% |
| IV | 69% |
| Fallopian tube carcinoma | |
|---|---|
| Stage | Relative five-year survival rate |
| I | 87% |
| II | 86% |
| III | 52% |
| IV | 40% |
| Low malignant potential tumors | |
|---|---|
| Stage | Relative five-year survival rate |
| I | 99% |
| II | 98% |
| III | 96% |
| IV | 77% |
Recurrence rates
Ovarian cancer frequently recurs after treatment. If a recurrence occurs in advanced disease, it typically occurs within 18 months of initial treatment (18 months progression-free survival). Recurrences can be treated, but the disease-free interval tends to shorten and chemoresistance increases with each recurrence. Low malignant potential tumors rarely relapse, even when fertility-sparing surgery is the treatment of choice. 15% of LMP tumors relapse after unilateral surgery in the previously unaffected ovary, and they are typically easily treated with surgery. More advanced tumors may take up to 20 years to relapse, if they relapse at all, and are only treated with surgery unless the tumor has changed its histological characteristics or grown very quickly. In these cases, and when there is significant ascites, chemotherapy may also be used.
Epidemiology

Globally, as of 2010, about 160,000 people died from ovarian cancer, up from 113,000 in 1990. As of 2014, more than 220,000 diagnoses of epithelial ovarian cancer were made yearly. In 2010, in the United States, an estimated 21,880 new cases were diagnosed and 13,850 women died of ovarian cancer. Around 1800 of the new diagnoses were sex-cord or stromal tumors. In the United Kingdom as of 2014, 7,000 yearly diagnoses were made and 4,200 deaths occurred. It is the 5th most common cancer in UK women.
The overall lifetime risk is around 1.6%. The risk in the UK is similar, at 1.7% (one woman in 60). Ashkenazi Jewish women carry mutated BRCA alleles at a rate five times that of the rest of the population, putting them at higher risk for ovarian cancer.
In the US, ovarian cancer affects 1.3-1.4% and is the cause of death of about 1% of women. This made it the fifth-leading cause of cancer-related deaths with an estimated 15,000 deaths in 2008. It occurs more commonly in developed countries. Ovarian cancer is the fifth-most common cancer in women in the UK (around 7,100 women were diagnosed with the disease in 2011), and it is the fifth-most common cause of cancer death in women (around 4,300 women died in 2012). It is the most deadly gynecologic cancer. In 2014, the incidence rate for women in developed countries was about 9.4 per 100,000, compared to 5.0 per 100,000 in developing countries.
The rate of ovarian cancer between 1993 and 2008 decreased in women of the 40-49 age cohort and in the 50-64 age cohort, possibly due to this group's widespread adoption of oral contraceptives. This decrease made it the ninth-most common cancer in women.
Society and culture
Other animals
Ovarian tumors have been reported in mares. Reported tumor types include teratoma, cystadenocarcinoma, and particularly granulosa cell tumor.
Research
Researchers are assessing different ways to screen for ovarian cancer. Screening tests that could potentially be used alone or in combination for routine screening include the CA-125 marker and transvaginal ultrasound. Doctors can measure the levels of the CA-125 protein in a woman’s blood; high levels could be a sign of ovarian cancer, but this is not always the case, and not all women with ovarian cancer have high CA-125 levels. Transvaginal ultrasound involves using an ultrasound probe to scan the ovaries from inside the vagina, giving a clearer image than scanning the abdomen. The UK Collaborative Trial of Ovarian Cancer Screening is testing a screening technique that combines CA-125 blood tests with transvaginal ultrasound. Several large studies are going on, but none has identified an effective technique. In 2009, however, early results from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) showed that a technique combining annual CA-125 tests with ultrasound imaging did help to detect the disease at an early stage. However, it's not yet clear if this approach could actually help to save lives—the full results of the trial will be published in 2015. One major problem with screening is no clear progression of the disease from stage I (noninvasive) to stage III (invasive) is seen, and it may not be possible to find cancers before they reach stage III. Another problem is that screening methods tend to find too many suspicious lesions, most of which are not cancer, but malignancy can only be assessed with surgery. The ROCA method combined with transvaginal ultrasonography is being researched in high-risk women to determine if it is a viable screening method. It is also being investigated in normal-risk women as it has shown promise in the wider population.
Research into various prognostic factors for ovarian cancer is also going on. Recent research shows that thrombocytosis predicts lower survival and higher stage cancer.
While an active area of research, no immunotherapy has been shown to be effective as of 2013. However, trials of the antibody and VEGF inhibitor bevacizumab, which can slow the growth of new blood vessels in the cancer, have shown promising results, especially in combination with pazopanib, which also slows the process of blood vessel growth. Bevacizumab has been particularly effective in preliminary studies on stage-III and -IV cancer and has been cited as having at least a 15% response rate. Angiogenesis inhibitors in the receptor tyrosine kinase inhibitor group, including pazopanib, cediranib, and nintedanib, have also been shown to increase progression free survival (PFS), but their benefit for overall survival has not been investigated as of yet. Bevacizumab can also be combined with platinum chemotherapy, a combination that has had positive preliminary results in PFS, but equivocal results regarding overall survival. One disadvantage to these treatments is the side effect profile, which includes high blood pressure and proteinuria. The drug can also exacerbate bowel disease, leading to fistulae or bowel perforation. Vintafolide, which consists of an antifolate conjugated with vinblastine, is also in clinical trials; it may prove beneficial because folate receptors are overexpressed in many ovarian cancers. Another potential immunotherapy is trastuzumab (Herceptin), which is active against tumors positive for Her2/neu mutations.
Intraperitoneal chemotherapy has also been under investigation during the 2000s and 2010s for its potential to deliver higher doses of cytotoxic agent to tumors. Preliminary trials with cisplatin and paclitaxel have shown it is not well tolerated, but does improve survival, and more tolerable regimens are being researched.
PARP inhibitors have also shown promise in early trials, particularly in people with BRCA gene mutations, since the BRCA protein interacts with the PARP pathway. It is also being studied in recurrent ovarian cancer in general, where preliminary studies have shown longer PFS. Specifically, olaparib has shown greater survival compared to doxorubicin, though this treatment is still being investigated. It is not clear yet which biomarkers are predictive of responsiveness to PARP inhibitors.
mTOR inhibitors were a highly investigated potential treatment in the 2000s and 2010s, but the side effects of these drugs (particularly hyperglycemia and hyperlipidemia) were not well tolerated and the survival benefit not confirmed. PI3 kinase inhibitors have been of interest, but they tend to be highly toxic and cause diarrhea. Another investigated drug is selumetinib, a MAPK inhibitor. It improved survival, but did not correlate with any mutations found in tumors.
References
- ^ "Ovarian Cancer Prevention (PDQ®)". NCI. December 6, 2013. Retrieved 1 July 2014.
- "Defining Cancer". National Cancer Institute. Retrieved 10 June 2014.
- ^ "Ovarian Epithelial Cancer Treatment (PDQ®)". NCI. 2014-05-12. Retrieved 1 July 2014.
- Ruddon, Raymond W. (2007). Cancer biology (4th ed. ed.). Oxford: Oxford University Press. p. 223. ISBN 9780195175431.
{{cite book}}:|edition=has extra text (help) - ^ World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.12. ISBN 9283204298.
- ^ "Ovarian Cancer Prevention (PDQ®)". NCI. 2014-06-20. Retrieved 1 July 2014.
- Piek JM, van Diest PJ, Verheijen RH (2008). "Ovarian carcinogenesis: an alternative hypothesis". Adv. Exp. Med. Biol. Advances in Experimental Medicine and Biology. 622: 79–87. doi:10.1007/978-0-387-68969-2_7. ISBN 978-0-387-68966-1. PMID 18546620.

- Moyer VA (Dec 18, 2012). "Screening for ovarian cancer: U.S. Preventive Services Task Force reaffirmation recommendation statement". Annals of internal medicine. 157 (12): 900–4. doi:10.7326/0003-4819-157-11-201212040-00539. PMID 22964825.
- "SEER Stat Fact Sheets: Ovary Cancer". NCI. Retrieved 18 June 2014.
- ^ Jayson GC, Kohn EC, Kitchener HC, Ledermann JA (October 2014). "Ovarian cancer". Lancet. 384 (9951): 1376–88. doi:10.1016/S0140-6736(13)62146-7. PMID 24767708.
- ^ Seiden, Michael V. (2012). "Gynecologic Malignancies". In Longo DL, Kasper DL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J (ed.). Harrison's Principles of Internal Medicine (18th ed.). McGraw-Hill. ISBN 978-0-07-174889-6.
{{cite book}}: CS1 maint: multiple names: editors list (link) - Goff BA (June 2012). "Ovarian cancer: screening and early detection". Obstetrics and gynecology clinics of North America. 39 (2): 183–94. doi:10.1016/j.ogc.2012.02.007. PMID 22640710.
- ^ Hoffman, Barbara L.; Schorge, John O.; Schaffer, Joseph I.; Halvorson, Lisa M.; Bradshaw, Karen D.; Cunningham, F. Gary (2012). "Epithelial Ovarian Cancer". Williams Gynecology (2nd ed.). McGraw Hill Medical. pp. 853–878. ISBN 978-0-07-171672-7.
- ^ DeCherney, Alan; Nathan, Lauren; Goodwin, T. Murphy; Laufer, Neri; Roman, Ashley (2012). "Pediatric and Adolescent Gynecology". Current Diagnosis & Treatment Obstetrics & Gynecology (11th ed.). ISBN 978-0071638562.
- Hippisley-Cox J, Coupland C (Jan 4, 2011). "Identifying women with suspected ovarian cancer in primary care: derivation and validation of algorithm". BMJ (Clinical research ed.). 344: d8009. doi:10.1136/bmj.d8009. PMC 3251328. PMID 22217630.
- Manson, JoAnn E.; Bassuk, Shari S. (2012). "The Menopause Transition and Postmenopausal Hormone Therapy". In Longo DL, Kasper DL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J (ed.). Harrison's Principles of Internal Medicine (18th ed.). McGraw-Hill. ISBN 978-0-07-174889-6.
{{cite book}}: CS1 maint: multiple names: editors list (link) - "Ovarian Cancer Prevention (PDQ®)". National Cancer Institute. 2013. Archived from the original on 2013-12-30. Retrieved 2013-12-30.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - Hjartåker A, Meo MS, Weiderpass E (January 2010). "Alcohol and gynecological cancers: an overview". European Journal of Cancer Prevention. 19 (1): 1–10. doi:10.1097/CEJ.0b013e328333fb3a. PMID 19926999.
- Cite error: The named reference
CRUKRiskswas invoked but never defined (see the help page). - ^ Odunsi, Kunle; Pejovic, Tanja; Anderson, Matthew L. (2011). Molecular Biology of Gynecologic Cancers. Wolters Kluwer/Lippincott Williams & Wilkins. pp. 1302–1310. ISBN 978-1-4511-0545-2.
{{cite book}}:|work=ignored (help) - "Genetics of Breast and Ovarian Cancer (PDQ®)". NCI. 2 October 2014. Retrieved 27 October 2014.
- Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS (2010-01-28). "Predictive Value of Symptoms for Early Detection of Ovarian Cancer". J Natl Cancer Inst. 102 (4): 222–9. doi:10.1093/jnci/djp500. PMC 2826180. PMID 20110551.
- ^ Miller RW, Ueland FR (March 2012). "Risk of malignancy in sonographically confirmed ovarian tumors". Clinical obstetrics and gynecology. 55 (1): 52–64. doi:10.1097/GRF.0b013e31824970cf. PMID 22343229.
- Dunn, J. D. (Ed.). Associated Title(s): PROTEOMICS – Clinical Applications. Vol. 11. Online ISSN: 1615-9861.
- "Guideline CG122. Ovarian cancer: The recognition and initial management of ovarian cancer, Appendix D: Risk of malignancy index (RMI I)". NICE clinical guidelines. April 2011.
- ^ Kosary, Carol L. (2007). "Chapter 16: Cancers of the Ovary". In Baguio, RNL; Young, JL; Keel, GE; Eisner, MP; Lin, YD; Horner, M-J (eds.). SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988-2001, Patient and Tumor Characteristics. SEER Program. Vol. NIH Pub. No. 07-6215. Bethesda, MD: National Cancer Institute. pp. 133–144.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ "Ovarian Cancer Staging" (PDF). Society for Gynecologic Oncology. 1 January 2014.
- "How is ovarian cancer staged?". Retrieved July 27, 2010.
- ^ "Diagnosis and Staging". Retrieved July 27, 2010.
- ^ Croswell, Jennifer M.; Brawley, Otis W.; Kramer, Barnett S. (2012). "Prevention and Early Detection of Cancer". In Longo DL, Kasper DL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J (ed.). Harrison's Principles of Internal Medicine (18th ed.). McGraw-Hill. ISBN 978-0-07-174889-6.
{{cite book}}: CS1 maint: multiple names: editors list (link) - Yao, Stephanie (19 December 2014). "FDA approves Lynparza to treat advanced ovarian cancer: First LDT companion diagnostic test also approved to identify appropriate patients". U.S. Food and Drug Administration.
- "Ovarian Cancer Treatments Available". Archived from the original on 2010-08-27. Retrieved July 27, 2010.
- "Follow up for ovarian cancer". Cancer Research UK.
- ^ Follow-up care from American Cancer Society. Last Medical Review: 03/21/2013. Last Revised: 02/06/2014
- ^ Society of Gynecologic Oncology (February 2014), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, Society of Gynecologic Oncology, retrieved 19 February 2013, which cites
- Bhosale P, Peungjesada S, Wei W, Levenback CF, Schmeler K, Rohren E, Macapinlac HA, Iyer RB (August 2010). "Clinical Utility of Positron Emission Tomography/Computed Tomography in the Evaluation of Suspected Recurrent Ovarian Cancer in the Setting of Normal CA-125 Levels". International Journal of Gynecological Cancer. 20 (6): 936–944. doi:10.1111/IGC.0b013e3181e82a7f. PMID 20683399.
- "ASCO Provisional Clinical Opinion: The Integration of Palliative Care into Standard Oncology Care". ASCO. Retrieved 20 August 2014.
- Radwany SM, von Gruenigen VE (Mar 2012). "Palliative and end-of-life care for patients with ovarian cancer". Clinical obstetrics and gynecology. 55 (1): 173–84. doi:10.1097/grf.0b013e31824b1af1. PMID 22343236.
- "Survival rates for ovarian cancer". American Cancer Society. April 22, 2013. Retrieved 2013-12-30.
- Society of Gynecologic Oncology (February 2014), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, Society of Gynecologic Oncology, retrieved 19 February 2013, which cites
- Smith TJ, Temin S, Alesi ER, Abernethy AP, Balboni TA, Basch EM, Ferrell BR, Loscalzo M, Meier DE, Paice JA, Peppercorn JM, Somerfield M, Stovall E, Von Roenn JH (6 February 2012). "American Society of Clinical Oncology Provisional Clinical Opinion: The Integration of Palliative Care Into Standard Oncology Care". Journal of Clinical Oncology. 30 (8): 880–887. doi:10.1200/JCO.2011.38.5161. PMID 22312101.
- Rezk Y, Timmins PF, Smith HS (26 December 2010). "Review Article: Palliative Care in Gynecologic Oncology". American Journal of Hospice and Palliative Medicine. 28 (5): 356–374. doi:10.1177/1049909110392204. PMID 21187291.
- Lewin SN, Buttin BM, Powell MA, Gibb RK, Rader JS, Mutch DG, Herzog TJ (November 2005). "Resource utilization for ovarian cancer patients at the end of life: How much is too much?". Gynecologic Oncology. 99 (2): 261–266. doi:10.1016/j.ygyno.2005.07.102. PMID 16140364.
- ^ Johannes, Laura (March 9, 2010). "Test to Help Determine If Ovarian Masses Are Cancer". The Wall Street Journal. Archived from the original on 2013-12-30. Retrieved 2013-12-30.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)
- ^ Survival rates based on SEER incidence and NCHS mortality statistics, as cited by the National Cancer Institute in SEER Stat Fact Sheets — Cancer of the Ovary
- Gucalp, Rasim; Dutcher, Janice (2012). "Oncologic Emergencies". In Longo DL, Kasper DL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J (ed.). Harrison's Principles of Internal Medicine (18th ed.). McGraw-Hill. ISBN 978-0-07-174889-6.
{{cite book}}: CS1 maint: multiple names: editors list (link) - "Survival rates for ovarian cancer, by stage". American Cancer Society. Retrieved 29 October 2014.
- "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved November 11, 2009.
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA (Dec 15, 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
- "Ovarian cancer risks and causes". Cancer Research UK. 15 January 2014. Retrieved 29 January 2015.
- ^ Ramirez, Pedro T.; Gershenson, David M. (September 2013). "Ovarian Cancer". The Merck Manual for Health Care Professionals.

- "Ovarian cancer statistics". Cancer Research UK. Retrieved 28 October 2014.
- Catone G, Marino G, Mancuso R, Zanghì A (April 2004). "Clinicopathological features of an equine ovarian teratoma". Reprod. Domest. Anim. 39 (2): 65–9. doi:10.1111/j.1439-0531.2003.00476.x. PMID 15065985.
- Lefebvre R, Theoret C, Doré M, Girard C, Laverty S, Vaillancourt D (November 2005). "Ovarian teratoma and endometritis in a mare". Can. Vet. J. 46 (11): 1029–33. PMC 1259148. PMID 16363331.
- Son YS, Lee CS, Jeong WI, Hong IH, Park SJ, Kim TH, Cho EM, Park TI, Jeong KS (May 2005). "Cystadenocarcinoma in the ovary of a Thoroughbred mare". Aust. Vet. J. 83 (5): 283–4. doi:10.1111/j.1751-0813.2005.tb12740.x. PMID 15957389.
- Frederico LM, Gerard MP, Pinto CR, Gradil CM (May 2007). "Bilateral occurrence of granulosa-theca cell tumors in an Arabian mare". Can. Vet. J. 48 (5): 502–5. PMC 1852596. PMID 17542368.
- Hoque S, Derar RI, Osawa T, Taya K, Watanabe G, Miyake Y (June 2003). "Spontaneous repair of the atrophic contralateral ovary without ovariectomy in the case of a granulosa theca cell tumor (GTCT) affected mare" ( – ). J. Vet. Med. Sci. 65 (6): 749–51. doi:10.1292/jvms.65.749. PMID 12867740.
{{cite journal}}: External link in|format= - Sedrish SA, McClure JR, Pinto C, Oliver J, Burba DJ (November 1997). "Ovarian torsion associated with granulosa-theca cell tumor in a mare". J. Am. Vet. Med. Assoc. 211 (9): 1152–4. PMID 9364230.
- Moll HD, Slone DE, Juzwiak JS, Garrett PD (1987). "Diagonal paramedian approach for removal of ovarian tumors in the mare". Vet Surg. 16 (6): 456–8. doi:10.1111/j.1532-950X.1987.tb00987.x. PMID 3507181.
- Doran R, Allen D, Gordon B (January 1988). "Use of stapling instruments to aid in the removal of ovarian tumours in mares". Equine Vet. J. 20 (1): 37–40. doi:10.1111/j.2042-3306.1988.tb01450.x. PMID 2835223.
- Partridge E, Kreimer AR, Greenlee RT, Williams C, Xu JL, Church TR, Kessel B, Johnson CC, Weissfeld JL, Isaacs C, Andriole GL, Ogden S, Ragard LR, Buys SS (April 2009). "Results from four rounds of ovarian cancer screening in a randomized trial". Obstet Gynecol. 113 (4): 775–82. doi:10.1097/AOG.0b013e31819cda77. PMC 2728067. PMID 19305319.
- Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, Lewis S, Davies S, Philpott S, Lopes A, Godfrey K, Oram D, Herod J, Williamson K, Seif MW, Scott I, Mould T, Woolas R, Murdoch J, Dobbs S, Amso NN, Leeson S, Cruickshank D, McGuire A, Campbell S, Fallowfield L, Singh N, Dawnay A, Skates SJ, Parmar M, Jacobs I (April 2009). "Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS)". Lancet Oncol. 10 (4): 327–40. doi:10.1016/S1470-2045(09)70026-9. PMID 19282241.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - Leffers N, Daemen T, Helfrich W, Boezen HM, Cohlen BJ, Melief CJ, Nijman HW (Sep 17, 2014). "Antigen-specific active immunotherapy for ovarian cancer". The Cochrane database of systematic reviews. 9: CD007287. doi:10.1002/14651858.CD007287.pub3. PMID 25229990.
Further reading
- Cannistra SA (December 2004). "Cancer of the ovary". N. Engl. J. Med. 351 (24): 2519–29. doi:10.1056/NEJMra041842. PMID 15590954.
External links
- Ovarian Cancer at American Cancer Society
- GeneReviews/NCBI/NIH/UW entry on BRCA1 and BRCA2 Hereditary Breast/Ovarian Cancer
- Interactive Health Tutorials Medline Plus: Ovarian cancer Using animated graphics and you can also listen to the tutorial
- UK statistics for ovarian cancer
- Patient information about ovarian cancer from Cancer Research UK
- What is Ovarian Cancer Infographic, information on ovarian cancer - Mount Sinai Hospital, New York