| Revision as of 18:35, 5 July 2014 editBoghog (talk | contribs)Autopatrolled, Extended confirmed users, IP block exemptions, New page reviewers, Pending changes reviewers, Rollbackers, Template editors137,551 edits →Contraindications: reformatted bare url with cite web template← Previous edit | Revision as of 18:35, 5 July 2014 edit undoBoghog (talk | contribs)Autopatrolled, Extended confirmed users, IP block exemptions, New page reviewers, Pending changes reviewers, Rollbackers, Template editors137,551 edits →Contraindications: added FDA warning about using Lidocaine Viscous to treat tooth painNext edit → | ||
| Line 89: | Line 89: | ||
| * ]<ref name="MedWatch_Lidocaine_HCl">{{cite web | url = http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm342035.htm | title = Lidocaine Hydrochloride and 5% Dextrose Injection | work = Safety Labeling Changes | publisher = FDA Center for Drug Evaluation and Research (CDER) | date = January 2014 }}</ref> | * ]<ref name="MedWatch_Lidocaine_HCl">{{cite web | url = http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm342035.htm | title = Lidocaine Hydrochloride and 5% Dextrose Injection | work = Safety Labeling Changes | publisher = FDA Center for Drug Evaluation and Research (CDER) | date = January 2014 }}</ref> | ||
| * ]<ref name="MedWatch_Lidocaine_HCl"/> | * ]<ref name="MedWatch_Lidocaine_HCl"/> | ||
| * Lidocaine Viscous should not be used to treat tooth pain in children and infants.<ref name="MedWatch_Lidocaine_Viscous">{{cite web | url = http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm402790.htm | title = Lidocaine Viscous: Drug Safety Communication - Boxed Warning Required - Should Not Be Used to Treat Teething Pain | publisher = FDA Center for Drug Evaluation and Research (CDER) | date = June 2014 }}</ref> | |||
| Exercise caution in patients with any of the following | Exercise caution in patients with any of the following | ||
Revision as of 18:35, 5 July 2014
Pharmaceutical compound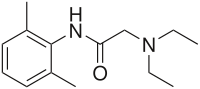 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xylocaine |
| Other names | N-(2,6-dimethylphenyl)-N,N-diethylglycinamide |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | intravenous, subcutaneous, topical, oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 35% (oral) 3% (topical) |
| Metabolism | Hepatic, 90% CYP1A2-mediated |
| Elimination half-life | 1.5–2 hours |
| Excretion | renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.821 |
| Chemical and physical data | |
| Formula | C14H22N2O |
| Molar mass | 234.34 g/mol g·mol |
| 3D model (JSmol) | |
| Melting point | 68 °C (154 °F) |
SMILES
| |
InChI
| |
| (verify) | |
Lidocaine (INN, BAN) /ˈlaɪdkeɪn/, xylocaine, or lignocaine (AAN, former BAN) /ˈlɪɡnkeɪn/ is a common local anesthetic and class 1b antiarrhythmic drug. Lidocaine is used topically to relieve itching, burning and pain from skin inflammations, injected as a dental anesthetic or as a local anesthetic for minor surgery.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medications needed in a basic healthcare system.
Medical uses
The efficacy profile of lidocaine as a local anesthetic is characterized by a rapid onset of action and intermediate duration of efficacy. Therefore, lidocaine is suitable for infiltration, block and surface anesthesia. Longer-acting substances such as bupivacaine are sometimes given preference for subdural and epidural anesthesias; lidocaine, on the other hand, has the advantage of a rapid onset of action. Epinephrine (aka adrenaline) vasoconstricts arteries reducing bleeding and also delays the resorption of lidocaine, almost doubling the duration of anaesthesia. For surface anesthesia several formulations are available that can be used e.g. for endoscopies, before intubations etc. Buffering the pH of lidocaine makes local freezing less painful. Lidocaine drops can be used on the eyes for short ophthalmic procedures.
Topical lidocaine has been shown in some patients to relieve the pain of postherpetic neuralgia (a complication of shingles), though there is not enough study evidence to recommend it as a first-line treatment. IV lidocaine also has uses as a temporary fix for tinnitus. Although not completely curing the disorder, it has been shown to reduce the effects by around two thirds.
Lidocaine is also the most important class 1B antiarrhythmic drug: it is used intravenously for the treatment of ventricular arrhythmias (for acute myocardial infarction, digoxin poisoning, cardioversion or cardiac catheterization) if amiodarone is not available or contraindicated. Lidocaine should be given for this indication after defibrillation, CPR, and vasopressors have been initiated.
A routine prophylactic administration is no longer recommended for acute cardiac infarction; the overall benefit of this measure is not convincing.
Inhaled lidocaine can be used as an antitussive (cough suppressor) acting peripherally to reduce the cough reflex. This application can be implemented as a safety and com for measure for patients that have to be intubated as it reduces the incidence of coughing and any tracheal damage it might cause when emerging from anesthesia.
Lidocaine along with ethanol, ammonia, and acetic acid has also been proven to be effective in treating jellyfish stings, both numbing the affected area and preventing further nematocyst discharge.
Insensitivity
Relative insensitivity to lidocaine is genetic. In hypokalemic sensory overstimulation, relative insensitivity to lidocaine has been described in people who also have attention deficit hyperactivity disorder. In dental anesthesia, a relative insensitivity to lidocaine can occur for anatomical reasons due to unexpected positions of nerves. Some people with Ehlers-Danlos syndrome are insensitive to lidocaine.
Contraindications
Absolute contraindications for the use of lidocaine include:
- Heart block, second or third degree (without pacemaker)
- Severe sinoatrial block (without pacemaker)
- Serious adverse drug reaction to lidocaine or amide local anesthetics
- Hypersensitivity to corn and corn-related products (corn-derived dextrose is used in the premixed injections)
- Concurrent treatment with quinidine, flecainide, disopyramide, procainamide (Class I antiarrhythmic agents)
- Prior use of amiodarone hydrochloride
- Adams-Stokes syndrome
- Wolff-Parkinson-White Syndrome
- Lidocaine Viscous should not be used to treat tooth pain in children and infants.
Exercise caution in patients with any of the following
- Hypotension not due to arrhythmia
- Bradycardia
- Accelerated idioventricular rhythm
- Elderly patients
- Pseudocholinesterase deficiency
- Intra-articular infusion (this is not an approved indication and can cause chondrolysis)
- Porphyria, especially Acute Intermittent Porphyria (AIP); lidocaine has been classified as porphyrogenic because of the hepatic enzymes it induces; although clinical evidence suggests that it is not. Bupivacaine is a safe alternative in this case.
- Impaired liver function - people with lowered hepatic function may have an adverse reaction with repeated administration of lidocaine because the drug is metabolized by the liver. Adverse reactions may include neurological symptoms (e.g. dizziness, nausea, muscle twitches, vomiting, or seizures).
Adverse effects
Adverse drug reactions (ADRs) are rare when lidocaine is used as a local anesthetic and is administered correctly. Most ADRs associated with lidocaine for anesthesia relate to administration technique (resulting in systemic exposure) or pharmacological effects of anesthesia, and allergic reactions only rarely occur. Systemic exposure to excessive quantities of lidocaine mainly result in central nervous system (CNS) and cardiovascular effects – CNS effects usually occur at lower blood plasma concentrations and additional cardiovascular effects present at higher concentrations, though cardiovascular collapse may also occur with low concentrations. ADRS are listed below by system:
CNS excitation: nervousness, agitation, anxiety, apprehension, tingling around the mouth (circumoral paraesthesia), headache, hyperesthesia, tremor, dizziness, pupillary changes, psychosis, euphoria, hallucinations, and seizures
CNS depression with increasingly heavier exposure: drowsiness, lethargy, slurred speech, hypoesthesia, confusion, disorientation, loss of consciousness, respiratory depression and apnoea.
Cardiovascular: hypotension, bradycardia, arrhythmias, flushing, venous insufficiency, increased defibrillator threshold, edema, and/or cardiac arrest – some of which may be due to hypoxemia secondary to respiratory depression.
Respiratory: Bronchospasm, dyspnea, respiratory depression or arrest
Gastrointestinal: metallic taste, nausea, vomiting
Ears: tinnitus
Eyes: local burning, Conjunctival hyperemia, corneal epithelial changes/ulceration, diplopia, visual changes (opacification)
Skin: itching, depigmentation, rash, urticaria, edema, angioedema, bruising, inflammation of the vein at the injection site, irritation of the skin when applied topically
Blood: methemoglobinemia
Allergic
ADRs associated with the use of intravenous lidocaine are similar to toxic effects from systemic exposure above. These are dose-related and more frequent at high infusion rates (≥3 mg/minute). Common ADRs include: headache, dizziness, drowsiness, confusion, visual disturbances, tinnitus, tremor, and/or paraesthesia. Infrequent ADRs associated with the use of lidocaine include: hypotension, bradycardia, arrhythmias, cardiac arrest, muscle twitching, seizures, coma, and/or respiratory depression.
Traditionally physicians have always advocated against using epinephrine with local anesthesia in end arterial structures (fingers, toes, nose, and penis) because vasospasm combined with a lack of collateral circulation in these areas may result in tissue necrosis. No clinical evidence has implicated lidocaine in particular as a cause of this adverse reaction.
Overdosage
Overdosage with lidocaine can be a result of excessive administration via topical or parenteral routes, accidental oral ingestion of topical preparations by children who are more susceptible to overdose, accidental intravenous (rather than subcutaneous, intrathecal or paracervical) injection or prolonged use of subcutaneous infiltration anesthesia during cosmetic surgical procedures. These occurrences have often led to severe toxicity or death in both children and adults. Lidocaine and its two major metabolites may be quantified in blood, plasma or serum to confirm the diagnosis in potential poisoning victims or to assist in the forensic investigation in a case of fatal overdosage. It is important in the interpretation of analytical results to recognize that lidocaine is often routinely administered intravenously as an antiarrhythmic agent in critical cardiac care situations. Treatment with intravenous lipid emulsions (used for parental feeding) to reverse the effects of local anaesthetic toxicity is becoming more commonplace.
Interactions
Any drugs that are also ligands of CYP3A4 and CYP1A2 can potentially increase serum levels and potential for toxicity or decrease serum levels and the efficacy depending on whether they induce or inhibit the enzymes respectively. Drugs that may increase the chance of methemoglobinemia should also be considered carefully. Dronedarone and liposomal morphine are both absolutely contraindicated as they may increase the serum levels but there are hundreds of other drugs that have to be monitored for interaction.
Dosage forms



Lidocaine, usually in the form of lidocaine hydrochloride, is available in various forms including:
- Injected local anesthetic (sometimes combined with epinephrine to reduce bleeding)
- Dermal patch (sometimes combined with prilocaine)
- Intravenous injection
- Intravenous infusion
- Intraosseous infusion
- Nasal instillation/spray (combined with phenylephrine)
- Oral gel (often referred to as "viscous lidocaine" or abbreviated "lidocaine visc" or "lidocaine hcl visc" in pharmacology; used as teething gel)
- Oral liquid
- Oral and topical ointments, with and without flavoring, respectively
- Topical gel (as with Aloe vera gels that include lidocaine)
- Topical liquid
- Lidocaine HCl 2% Jelly, combined with hypromellose, to anesthetize and lubricate the urethra, etc., for inserting a catheter or instrument
- Topical patch (lidocaine 5%), marketed since 1999 in the US by Endo Pharmaceuticals as "Lidoderm" - and since 2007 in the UK by Grünenthal as "Versatis"
- Topical ointment (lidocaine 5%) as a temporary reliever of discomfort associated anorectal disorders, such as hemorrhoids, marketed as an over-the-counter product in the US as "RectiCare" since 2012 by Ferndale Healthcare, Inc.
- Topical aerosol spray
- Inhaled via a nebulizer
- As a component of a GI cocktail used in emergency rooms
- Ophthalmic solution
Adulterant in cocaine
Lidocaine is often added to cocaine as a diluent. Cocaine numbs the gums when applied, and since lidocaine causes stronger numbness, a user gets the impression of high-quality cocaine when in actuality, the user is receiving a diluted product.
Preparation
Lidocaine may be prepared in two steps by the reaction of 2,6-xylidine with chloroacetyl chloride, followed by the reaction with diethylamine:

Preparation of Lidocaine: U.S. patent 2,441,498, Bengt JL, Niels, ML, Astra Apotekarnes Kem Fab (1948).
Pharmacokinetics
The onset of action of lidocaine is about 45 to 90 seconds and its duration is 10 to 20 minutes. It is approximately 95% metabolized (dealkylated) in the liver mainly by CYP3A4 to the pharmacologically-active metabolites monoethylglycinexylidide (MEGX) and then subsequently to the inactive glycine xylidide. MEGX has a longer half life than lidocaine but also is a less potent sodium channel blocker. The volume of distribution is 1.1-2.1 L/kg but congestive heart failure can decrease it. 60-80% circulates bound to the protein alpha1 acid glycoprotein. The oral bioavailability is 35% and the topical bioavailability is 3%.
The elimination half-life of lidocaine is biphasic and approximately 90–120 minutes in most patients. This may be prolonged in patients with hepatic impairment (average 343 minutes) or congestive heart failure (average 136 minutes). Lidocaine is excreted in the urine (90% as metabolites and 10% as unchanged drug).
Pharmacodynamics
Anaesthesia
Lidocaine alters signal conduction in neurons by blocking the fast voltage gated sodium (Na) channels in the neuronal cell membrane that are responsible for signal propagation. With sufficient blockage the membrane of the postsynaptic neuron will not depolarize and will thus fail to transmit an action potential. This creates the anaesthetic effect by not merely preventing pain signals from propagating to the brain but by stopping them before they begin. Careful titration allows for a high degree of selectivity in the blockage of sensory neurons, whereas higher concentrations will also affect other modalities of neuron signaling.
Antiarrhythmic
The same principle applies for this drug's actions in the heart. Blocking sodium channels in the conduction system as well as the muscle cells of the heart raises the depolarization threshold making the heart less likely to initiate or conduct early action potentials that may cause an arrhythmia.
History
Lidocaine, the first amino amide–type local anesthetic, was first synthesized under the name xylocaine by Swedish chemist Nils Löfgren in 1943. His colleague Bengt Lundqvist performed the first injection anesthesia experiments on himself. It was first marketed in 1949.
Recreational use
Lidocaine is not currently listed by the World Anti-Doping Agency as an illegal substance. Lidocaine is used as an adjuvant, adulterant, and diluent to street drugs such as cocaine and heroin.
Compendial status
See also
- Lidocaine/prilocaine
- QX-314
- Dimethocaine (has some DRI activity)
- Procaine
Notes and references
- "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- Cepeda MS, Tzortzopoulou A, Thackrey M, Hudcova J, Arora Gandhi P, Schumann R (2010). "Adjusting the pH of lidocaine for reducing pain on injection". Cochrane Database Syst Rev (12): CD006581. doi:10.1002/14651858.CD006581.pub2. PMID 21154371.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Khaliq W, Alam S, Puri N (2007). "Topical lidocaine for the treatment of postherpetic neuralgia". Cochrane Database Syst Rev (2): CD004846. doi:10.1002/14651858.CD004846.pub2. PMID 17443559.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - "New hope for tinnitus sufferers". BBC News. 9 January 2008.
- Kalcioglu MT, Bayindir T, Erdem T, Ozturan O. (2005). "Objective evaluation of the effects of intravenous lidocaine on tinnitus". Hearing Research.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Adcock JJ, Douglas GJ, Garabette M, Gascoigne M, Beatch G, Walker M, Page CP (February 2003). "RSD931, a novel anti-tussive agent acting on airway sensory nerves". Br. J. Pharmacol. 138 (3): 407–16. doi:10.1038/sj.bjp.0705056. PMC 1573683. PMID 12569065.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Biller JA (2007). "Airway obstruction, bronchospasm, and cough". In Berger AM, Shuster JL, Von Roenn JH (ed.). Principles and practice of palliative care and supportive oncology. Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 297–307. ISBN 978-0-7817-9595-1.
Inhaled lidocaine is used to suppress cough during bronchoscopy. Animal studies and a few human studies suggest that lidocaine has an antitussive effect…
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: editors list (link) - Minogue, SC (Oct 2004). "Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia". Anesthesia and analgesia. 99 (4): 1253–7, table of contents. PMID 15385385.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Birsa LM, Verity PG, Lee RF (May 2010). "Evaluation of the effects of various chemicals on discharge of and pain caused by jellyfish nematocysts". Comp. Biochem. Physiol. C Toxicol. Pharmacol. 151 (4): 426–30. doi:10.1016/j.cbpc.2010.01.007. PMID 20116454.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Morabito, R (Jun 2014). "Nematocyst discharge in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms can be affected by lidocaine, ethanol, ammonia and acetic acid". Toxicon : official journal of the International Society on Toxinology. 83: 52–8. PMID 24637105.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Segal, MM (Dec 2007). "Hypokalemic sensory overstimulation". Journal of child neurology. 22 (12): 1408–10. PMID 18174562.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Hakim AJ, Grahame R, Norris P, Hopper C (February 2005). "Local anaesthetic failure in joint hypermobility syndrome". J R Soc Med. 98 (2): 84–5. doi:10.1258/jrsm.98.2.84. PMC 1079398. PMID 15684369.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Lidocaine Hydrochloride and 5% Dextrose Injection". Safety Labeling Changes. FDA Center for Drug Evaluation and Research (CDER). January 2014.
- "Lidocaine Viscous: Drug Safety Communication - Boxed Warning Required - Should Not Be Used to Treat Teething Pain". FDA Center for Drug Evaluation and Research (CDER). June 2014.
- "Table 96–4. Drugs and Porphyria" (PDF). Merck Manual. Merck & Company, Inc. 2011.
- "Lidocaine - N01BB02". Drug porphyrinogenicity monograph. The Norwegian Porphyria Centre (NAPOS) and The Swedish Porphyria Centre.
strong clinical evidence points to lidocaine as probably not porphyrinogenic
- Khan, M. Gabriel (2007). Cardiac Drug Therapy (7th ed. ed.). Totowa, NJ: Humana Press. ISBN 9781597452380.
{{cite book}}:|edition=has extra text (help) - Jackson D, Chen AH, Bennett CR (October 1994). "Identifying true lidocaine allergy". J Am Dent Assoc. 125 (10): 1362–6. PMID 7844301.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Australian Medicines Handbook. Adelaide, S. Aust: Australian Medicines Handbook Pty Ltd. 2006. ISBN 0-9757919-2-3.
- Chowdhry, S (Dec 2010). "Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases". Plastic and reconstructive surgery. 126 (6): 2031–4. PMID 20697319.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 840–4. ISBN 0-9626523-7-7.
- Picard J, Ward SC, Zumpe R, Meek T, Barlow J, Harrop-Griffiths W (February 2009). "Guidelines and the adoption of 'lipid rescue' therapy for local anaesthetic toxicity". Anaesthesia. 64 (2): 122–5. doi:10.1111/j.1365-2044.2008.05816.x. PMID 19143686.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - https://online.epocrates.com/u/104316/lidocaine/Drug+Interactions. Retrieved April 2014.
{{cite web}}: Check date values in:|accessdate=(help); Missing or empty|title=(help) - "Product information for lidocaine ointment, USP 5%, spearmint flavored" (PDF). Product Insert. Taro Pharmaceutical Industries Ltd. Retrieved July 27, 2009.
- "Lidocaine Ointment Prescribing Information". Drugs.com. Retrieved January 22, 2012.
- "Solarcaine". Schering-Plough Healthcare Products, Inc. Retrieved July 27, 2009.
- "Lidoderm (Lidocaine Patch 5%)". Our Products. Endo Pharmaceuticals. Retrieved 18 October 2012.
- Bernardo NP, Siqueira MEPB, De Paiva MJN, Maia PP (2003). "Caffeine and other adulterants in seizures of street cocaine in Brazil". International Journal of Drug Policy. 14 (4): 331–4. doi:10.1016/S0955-3959(03)00083-5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kimberly H (1997-12-15). "Take a big-picture approach when dealing with corneal sensation". Retrieved 2009-04-23.
Lidocaine is more potent, with rapid diffusion and penetration.
- "UNITED STATES of America, Plaintiff-Appellee, v. Luis A. CUELLO, Alvaro Bastides-Benitez, John Doe, a/k/a Hugo Hurtado, and Alvaro Carvajal, Defendants-Appellants". Docket No. 78-5314. United States Court of Appeals, Fifth Circuit. 1979-07-25.
- Reilly TJ (1999). "The Preparation of Lidocaine". Journal of Chemical Education. 76 (11): 1557. doi:10.1021/ed076p1557.
- US patent 2441498, Bengt JL, Niels, ML, "Alkyl glycinanilides", issued 1948-05-11, assigned to Astra Apotekarnes Kem FAB
- Lewin NA, Nelson LH (2006). "Chapter 61: Antidysrhythmics". In Flomenbaum N, Goldfrank LR, Hoffman RL, Howland MD, Lewin NA, Nelson LH (ed.). Goldfrank's Toxicologic Emergencies (8th ed.). New York: McGraw-Hill. pp. 963–4. ISBN 0-07-143763-0.
{{cite book}}: CS1 maint: multiple names: editors list (link) - Thomson PD, Melmon KL, Richardson JA, Cohn K, Steinbrunn W, Cudihee R, Rowland M (April 1973). "Lidocaine pharmacokinetics in advanced heart failure, liver disease, and renal failure in humans". Ann. Intern. Med. 78 (4): 499–508. doi:10.7326/0003-4819-78-4-499. PMID 4694036.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - COLLINSWORTH, KEN A., SUMNER M. KALMAN, and DONALD C. HARRISON. "The clinical pharmacology of lidocaine as an antiarrhythymic drug." Circulation 50.6 (1974): 1217-1230. PMID:
- Carterall, William A. (2001). "Sodium Channels and Neuronal Hyperexcitability". Novartis Foundation Symposia. 241: 206. doi:10.1002/0470846682.ch14. ISBN 9780470846681.
{{cite journal}}:|chapter=ignored (help); Cite journal requires|journal=(help) - Sheu, SS; Lederer, WJ (Oct 1985). "Lidocaine's negative inotropic and antiarrhythmic actions. Dependence on shortening of action potential duration and reduction of intracellular sodium activity". Circulation research. 57 (4): 578–90. PMID 2412723.
- ^ Löfgren N (1948). Studies on local anesthetics: Xylocaine: a new synthetic drug (Inaugural dissertation). Stockholm, Sweden: Ivar Heggstroms. OCLC 646046738.
- Löfgren N, Lundqvist B (1946). "Studies on local anaesthetics II". Svensk Kemisk Tidskrift. 58: 206–17.
- Wildsmith JAW (2011). "Lidocaine: A more complex story than 'simple' chemistry suggests" (PDF). The Proceedings of the History of Anaesthesia Society. 43: 9–16.
- "The 2010 Prohibited List International Standard" (PDF). The World Anti-Doping Code. World Anti-Doping Agency (WADA). 19 September 2009.
- "New York Drug Threat Assessment". National Drug Intelligence Center. November 2002.
- "Revision Bulletin: Lidocaine and Prilocaine Cream–Revision to Related Compounds Test". The United States Pharmacopeial Convention. November 30, 2007.
- Lim TK, Macleod BA, Ries CR, Schwarz SK (2007). "The quaternary lidocaine derivative, QX-314, produces long-lasting local anesthesia in animal models in vivo". Anesthesiology. 107 (2): 305–11.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- U.S. National Library of Medicine: Drug Information Portal - Lidocaine
- Endo Pharmaceuticals' Lidoderm website
| Antiarrhythmic agents (C01B) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Channel blockers |
| ||||||||||||
| Receptor agonists and antagonists |
| ||||||||||||
| Ion transporters |
| ||||||||||||
| |||||||||||||
| Vasoprotectives (C05) | |||||||
|---|---|---|---|---|---|---|---|
| Antihemorrhoidals for topical use |
| ||||||
| Antivaricose therapy |
| ||||||
| Capillary stabilising agents |
| ||||||
| Antipruritics (D04) | |
|---|---|
| Antihistamines for topical use | |
| Anesthetics for topical use | |
| Others |
|
| Local anesthetics (primarily sodium channel blockers) (N01B) | |||||||
|---|---|---|---|---|---|---|---|
| Esters by acid |
| ||||||
| Amides | |||||||
| Combinations | |||||||
| |||||||
| Throat preparations (R02) | |
|---|---|
| Antiseptics | |
| Antibiotics | |
| Local anesthetics | |
| Other | |
| Drugs used for diseases of the ear (S02) | |
|---|---|
| Infection | |
| Corticosteroids | |
| Analgesics and anesthetics | |