 | |
| Clinical data | |
|---|---|
| Other names | BMS-791325 |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
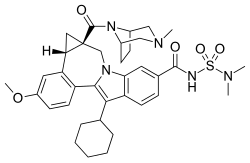
| Formula | C36H45N5O5S |
| Molar mass | 659.85 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Beclabuvir (also known by the research name BMS-791325; abbreviated BCV) is an antiviral drug for the treatment of hepatitis C virus (HCV) infection that has been studied in clinical trials. In February 2017, Bristol-Myers Squibb began sponsoring a post-marketing trial of beclabuvir, in combination with asunaprevir and daclatasvir, to study the combination's safety profile with regard to liver function. From February 2014 to November 2016, a phase II clinical trial was conducted on the combination of asunaprevir/daclatasvir/beclabuvir (beclabuvir is referred to as BMS-791325 in the trial) on patients infected with both HIV and HCV. Furthermore, a recent meta-analysis of six published six clinical trials showed high response rates in HCV genotype 1-infected patients treated with daclatasvir, asunaprevir, and beclabuvir irrespective of ribavirin use, prior interferon-based therapy, or restriction on noncirrhotic patients, IL28B genotype, or baseline resistance-associated variants
Pharmacology
Beclabuvir acts as a NS5B (RNA polymerase) inhibitor.
References
- Clinical trial number NCT03071133 for "Asunaprevir/Daclatasvir/Beclabuvir Fixed-Dose Combination Safety Surveillance in Japanese Patients With Chronic Hepatitis C (HCV) or Japanese Patients With Compensated Cirrhosis" at ClinicalTrials.gov
- Clinical trial number NCT02124044 for "Safety, Tolerability, and Efficacy of Asunaprevir and Daclatasvir in Subjects Coinfected With HIV-HCV" at ClinicalTrials.gov
- Ahmed AM, Doheim MF, Mattar OM, Sherif NA, Truong DH, Hoa PT, et al. (May 2018). "Beclabuvir in combination with asunaprevir and daclatasvir for hepatitis C virus genotype 1 infection: A systematic review and meta-analysis". Journal of Medical Virology. 90 (5): 907–918. doi:10.1002/jmv.24947. PMID 28892235. S2CID 3829214.
- Tatum H, Thuluvath PJ, Lawitz E, Martorell C, DeMicco M, Cohen S, et al. (August 2015). "A randomized, placebo-controlled study of the NS5B inhibitor beclabuvir with peginterferon/ribavirin for HCV genotype 1". Journal of Viral Hepatitis. 22 (8): 658–64. doi:10.1111/jvh.12372. PMID 25496007.
| RNA virus antivirals (primarily J05, also S01AD and D06BB) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C |
| ||||||||
| Hepatitis D | |||||||||
| Picornavirus | |||||||||
| Anti-influenza agents | |||||||||
| Multiple/general |
| ||||||||
| |||||||||
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |