 | |
| Clinical data | |
|---|---|
| Trade names | GS-6620 |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
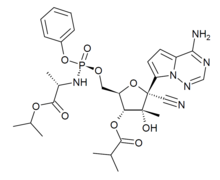
| Formula | C29H37N6O9P |
| Molar mass | 644.622 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
GS-6620 is an antiviral drug which is a nucleotide analogue. It was developed for the treatment of Hepatitis C but while it showed potent antiviral effects in early testing, it could not be successfully formulated into an oral dosage form due to low and variable absorption in the intestines which made blood levels unpredictable. It has however continued to be researched as a potential treatment for other viral diseases such as Ebola virus disease.
References
- Cho A, Zhang L, Xu J, Lee R, Butler T, Metobo S, et al. (March 2014). "Discovery of the first C-nucleoside HCV polymerase inhibitor (GS-6620) with demonstrated antiviral response in HCV infected patients". Journal of Medicinal Chemistry. 57 (5): 1812–25. doi:10.1021/jm400201a. PMID 23547794.
- Feng JY, Cheng G, Perry J, Barauskas O, Xu Y, Fenaux M, et al. (2014). "Inhibition of hepatitis C virus replication by GS-6620, a potent C-nucleoside monophosphate prodrug". Antimicrobial Agents and Chemotherapy. 58 (4): 1930–42. doi:10.1128/AAC.02351-13. PMC 4023746. PMID 24419349.
- Murakami E, Wang T, Babusis D, Lepist EI, Sauer D, Park Y, et al. (2014). "Metabolism and pharmacokinetics of the anti-hepatitis C virus nucleotide prodrug GS-6620". Antimicrobial Agents and Chemotherapy. 58 (4): 1943–51. doi:10.1128/AAC.02350-13. PMC 4023801. PMID 24419340.
- Gentile I, Coppola N, Buonomo AR, Zappulo E, Borgia G (September 2014). "Investigational nucleoside and nucleotide polymerase inhibitors and their use in treating hepatitis C virus". Expert Opinion on Investigational Drugs. 23 (9): 1211–23. doi:10.1517/13543784.2014.921680. PMID 24848437. S2CID 12115460.
- De Clercq E (March 2016). "C-Nucleosides To Be Revisited". Journal of Medicinal Chemistry. 59 (6): 2301–11. doi:10.1021/acs.jmedchem.5b01157. PMID 26513594.
- De Clercq E (November 2019). "New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections". Chemistry: An Asian Journal. 14 (22): 3962–3968. doi:10.1002/asia.201900841. PMC 7159701. PMID 31389664.
| RNA virus antivirals (primarily J05, also S01AD and D06BB) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C |
| ||||||||
| Hepatitis D | |||||||||
| Picornavirus | |||||||||
| Anti-influenza agents | |||||||||
| Multiple/general |
| ||||||||
| |||||||||
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |