Pharmaceutical compound
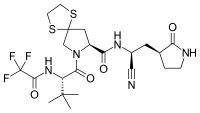 | |
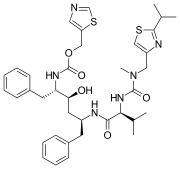 Chemical structures of simnotrelvir (top) and ritonavir (bottom) Chemical structures of simnotrelvir (top) and ritonavir (bottom) | |
| Combination of | |
|---|---|
| Simnotrelvir | SARS-CoV-2 3CL inhibitor |
| Ritonavir | Protease inhibitor |
| Clinical data | |
| Trade names | 先诺欣 (Xiannuoxin) |
| Pregnancy category |
|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Clinical data | |
|---|---|
| Other names | SIM0417, SSD8432 |
| Routes of administration | Oral |
| Drug class | SARS-CoV-2 3CL inhibitor |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 72.5% |
| Metabolism | hepatic (CYP3A) |
| Elimination half-life | 3.1 h; 4.1 h with ritonavir |
| Excretion | urine (55.4%), feces (36.7%) |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C22H30F3N5O4S2 |
| Molar mass | 549.63 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Simnotrelvir/ritonavir (trade name Xiannuoxin) is a pharmaceutical drug used for the treatment of COVID-19. Simnotrelvir/ritonavir is a combination drug of simnotrelvir, an inhibitor of SARS-CoV-2 3CL, and ritonavir, a CYP3A inhibitor.
It was developed by Simcere Pharmaceutical and conditionally approved in China by the National Medical Products Administration (NMPA) in January 2023. Results for the phase Ib trial are available. In a phase II/III trial, it reduced the duration of symptoms by a median of 36 hours compared to placebo.
See also
References
- ^ Simcere (January 28, 2023). "先诺欣 [先诺特韦片/利托那韦片组合包装 Simnotrelvir Tablets/Ritonavir Tablets(co-packaged)]". Dxy.cn package insert database (in Chinese (China)). Retrieved 2 October 2023.
- Zhu KW (September 2023). "Deuremidevir and Simnotrelvir-Ritonavir for the Treatment of COVID-19". ACS Pharmacology & Translational Science. 6 (9): 1306–1309. doi:10.1021/acsptsci.3c00134. PMC 10496140. PMID 37705591.
- Wang Q, Chen G, He J, Li J, Xiong M, Su H, et al. (May 2023). "Structure-Based Design of Potent Peptidomimetic Inhibitors Covalently Targeting SARS-CoV-2 Papain-like Protease". International Journal of Molecular Sciences. 24 (10): 8633. doi:10.3390/ijms24108633. PMC 10218254. PMID 37239980.
- "China approves two oral drugs to treat COVID-19". bioworld.com. January 30, 2023.
- Wang F, Xiao W, Tang Y, Cao M, Shu D, Asakawa T, et al. (September 2023). "Efficacy and safety of SIM0417 (SSD8432) plus ritonavir for COVID-19 treatment: a randomised, double-blind, placebo-controlled, phase 1b trial". The Lancet Regional Health. Western Pacific. 38: 100835. doi:10.1016/j.lanwpc.2023.100835. PMC 10362366. PMID 37484496.
- Cao B, Wang Y, Lu H, Huang C, Yang Y, Shang L, et al. (2024-01-18). "Oral Simnotrelvir for Adult Patients with Mild-to-Moderate Covid-19". New England Journal of Medicine. 390 (3): 230–241. doi:10.1056/NEJMoa2301425. PMC 11156186. PMID 38231624. S2CID 267030019.
External links
- NHSA slideshow, also shows adverse effect data from phase II/III
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |
| RNA virus antivirals (primarily J05, also S01AD and D06BB) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C |
| ||||||||
| Hepatitis D | |||||||||
| Picornavirus | |||||||||
| Anti-influenza agents | |||||||||
| Multiple/general |
| ||||||||
| |||||||||