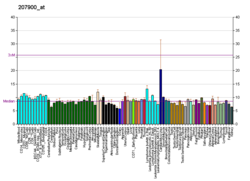
Mammalian protein found in Homo sapiens
CCL17 is a powerful chemokine produced in the thymus and by antigen-presenting cells like dendritic cells, macrophages, and monocytes. CCL17 plays a complex role in cancer. It attracts T-regulatory cells allowing for some cancers to evade an immune response. However, in other cancers, such as melanoma, an increase in CCL17 is linked to an improved outcome. CCL17 has also been linked to autoimmune and allergic diseases.
Classification
CCL17 (CC chemokine ligand 17) was initially named TARC (thymus- and activation-regulated chemokine) when first isolated in 1996. It was later renamed CCL17 as the naming conventions for all cytokines were updated to standardize names.
Function
Cytokines, like CCL17, help cells communicate with one another, and stimulate cell movement. Chemokines are a type of cytokine that attract white blood cells to sites of inflammation or disease. CCL17 as well as its partner chemokine CCL22 induce chemotaxis in T-helper cells. They do this by binding to CCR4, a chemokine receptor expressed on type 2 helper T cells, cutaneous lymphocyte skin-localizing T cells, and regulatory T cells. CCR4 is also expressed by T cells involved in adult T-cell leukemia/lymphoma and cutaneous T cell lymphomas, making its ligands (namely CCL17) an attractive target for novel therapies as described below. CCL17 is one of the few chemokines that are not stored in the body, except in the thymus; these chemokines are made when needed by dendritic cells, macrophages, and monocytes. CCL17 is expressed constitutively in the thymus, but only transiently in phytohemagglutinin-stimulated peripheral blood mononuclear cells. CCL17 can also be detected in other tissues such as the colon, small intestine, and lung. Granulocyte-macrophage colony-stimulating factor (GM-CSF) upregulates CCL17 production in monocytes and macrophages. Dendritic cells will produce large quantities of CCL17 when stimulated with IL-4 or TSLP.
CCL17 was the first CC chemokine identified that interacted with T cells with high affinity. CCL17 was also found to interact with monocytes, but with less affinity. It does not interact with granulocytes. It acts as a powerful chemoattractant to T-helper cells and T-regulatory cells because both can express CCR4.
Cancer
Classic Hodgkin lymphoma
CCL17 was found to be highly expressed by the tumor cells of classic Hodgkin lymphoma. It can be detected by immunohistochemistry in >90% of cases in a diagnostic setting and is highly specific within B cell derived cancers. CCL17 is mainly responsible for the presence of large amounts of T-helper and T-regulatory cells in the tumor microenvironment, which is considered a hallmark of Hodgkin lymphoma. Levels of CCL17 in serum are ~400 times higher in Hodgkin lymphoma patients than in healthy controls and are strongly associated with tumor volume, disease stage, and response to therapy. Its levels are increasing already several years prior to symptoms and diagnosis in many Hodgkin lymphoma patients.
Solid cancers
This chemokine is very important in the human body’s response to cancers. While it sometimes allows cancer to invade more rapidly, it more often helps the human body fight cancer. Some cancers that form tumors, such as breast cancer, produce CCL17 which draws T regulatory cells into the area, enhancing the cancer’s ability to invade. On the other hand, CCL17 will also activate tumor-infiltrating lymphocytes tumors. For many cancers, the more CCL17 in the area, the better the prognosis is for cancer survival or recovery.
Inflammation
Like many cytokines, CCL17 is inflammatory, so while it plays a largely helpful role in attacking cancers, it can induce inflammatory diseases, including allergic skin diseases. Because of its inflammatory effects, much of the medical research is on methods to mitigate CCL17. Neutralizing CCL17 with monoclonal antibodies has been shown to relieve inflammatory arthritis and osteoarthritis. Topical steroids have been found to be an effective tool in normalizing levels of CCL17.
Autoimmunity
CCL17 is known to help leukocytes (and especially eosinophils) target their response to skin-located pathogens. This often occurs through the CCL17-CCR4 interaction on type 2 T helper cells, which then secrete a variety of interleukins. Direct interactions between CCL17 and eosinophils has been observed but not well defined. However, overexpressed CCL17 has been linked to atopic dermatitis (eczema) and multiple sclerosis, among other autoimmune diseases. Studies have shown that children with allergies and atopic dermatitis have higher quantiles of CCL17 compared to children without allergies. As such, therapeutic approaches involving CCL17 regulation have shown some success in several cases. This intervention often involves interfering with CCR4 through monoclonal antibody treatment (such as mogamulizumab). Another option is small-molecule interaction with CCR4, which has not yet had any clinical success.
Atopic dermatitis (eczema)
Researchers have found that type 2 helper-T cells in lesions of atopic dermatitis (AD) express more IL-4 and IL-13 than unaffected Th2 cells. Dendritic cells respond to IL-4 and IL-13 by secreting CCL17 (as well as CCL18 and CCL22), especially in "barrier-disrupted" skin (such as lesional skin). Because CCL17 is a key attractant for Th2, this creates a cycle of Th2 recruitment, IL-4 and IL-13 signaling, dendritic cell secretion of CCL17, and further recruitment of Th2 cells. Severity of AD is therefore correlated with concentration of CCL17 and CCL22 in both the blood serum and interstitial fluid of pediatric and adult patients with either acute or chronic AD. Because Th2 cells are present at elevated levels during pregnancy, a buildup of CCL17 in umbilical cord blood may summon more Th2 cells, causing the aforementioned positive feedback loop. This is correlated with a higher likelihood of developing AD (and other allergic diseases) in infants (including for mothers without AD), especially for the first two years of infancy.
In adult patients, other signals (such as IL-22) have been shown to correlate with the severity and chronicity of AD in addition to levels of CCL17, although the causal relationships between each of these other signals and CCL17 are not all yet known. Other signaling components, like TSLP, are induced by other lesional epidermal cells and directly upregulate CCL17 production.
Clinically, CCL17 has recently shown promise as a useful biomarker for AD severity as well as efficacy of treatment. Historically, physicians have used mostly visual, qualitative evaluations of lesion progress, but using CCL17 to quantify AD has allowed for more precise and accurate records of progress (or regression) during treatment. In concert with this, proposed treatments for AD include topical regulation of CCL17. Especially for infantile AD, where prolonged AD has been linked to severe food allergies, early quantification and treatment is especially important. This treatment may take the form of small-molecule inhibition of CCL17-CCR4 binding, which inhibits recruitment of Th2 cells and subsequent development of lesions.
Multiple sclerosis (and EAE)
Multiple sclerosis (MS) (and the animal model EAE) are autoimmune diseases characterized in part by changes in the expression and regulation of CCL17 in cerebrospinal fluid. There is also evidence to suggest that certain SNPs in the CCL17 and CCL22 genes may raise the risk of MS for an individual.
While type 2 helper T (Th2) cells are a key component of AD because they are localized to the skin through the CCL17-CCR4 interaction, memory Th17 cells seem to express high levels of CCR4 in both human and murine models of MS and are therefore likely candidates for study and therapy.
Treatments of MS (such as natalizumab or methylprednisolone) seem to lower overall chemokine levels (notably including either CCL17 itself or factors that are known to induce CCL17 production) in addition to other purported primary functions. However, these findings are complicated by CCR4 up- and downregulation findings, which have sometimes seemed counter to the CCL17 localization pathways. Experimental explorations with CCL17-deficient mice have therefore counterintuitively given different information than experiments measuring CCR4 regulation for EAE.
Other disorders
Several other disorders are also correlated with high levels of CCL17 or use CCL17 to localize Th2 cells. CCL17 can act as an inflammatory agent or as a symptom, and in either case, disrupting or manipulating the expression or ligand binding offers a therapeutic target. And, regardless of therapeutic potential, it can be used as a biomarker of disease.
- Drug rash with eosinophilia and systemic symptoms (DRESS)
- Bullous pemphigoid (BP)
- Senile erythroderma
- Eosinophilic pustular folliculitis
- Chronic spontaneous urticaria (hives)
- Maculopapular exanthema
- Stevens-Johnson syndrome/toxic epidermal necrolysis
- (Non-)episodic angioedema with eosinophilia
- Allergic asthma
- Allergic rhinitis/chronic rhinosinusitis with nasal polyps (CRSwNP)
- Eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome)
- Acute and chronic eosinophilic pneumonia
- Mycosis fungoides (MF)
- Sezary syndrome (SS)
- Lymphocytic variant HES
- Acute disseminated encephalomyelitis (ADEM)
- Neuromyelitis optica (NMO) (Devic's disease)
Chromosomal location
In humans the gene for CCL17 is located on chromosome 16 along with other chemokines including CCL22 and CX3CL1.
References
- ^ GRCh38: Ensembl release 89: ENSG00000102970 – Ensembl, May 2017
- ^ GRCm38: Ensembl release 89: ENSMUSG00000031780 – Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Lacy P (2017). "Eosinophil Cytokines in Allergy". Cytokine Effector Functions in Tissues. Elsevier. pp. 173–218. doi:10.1016/b978-0-12-804214-4.00011-7. ISBN 978-0-12-804214-4.
- ^ Korbecki J, Kojder K, Simińska D, Bohatyrewicz R, Gutowska I, Chlubek D, Baranowska-Bosiacka I (November 2020). "CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4". International Journal of Molecular Sciences. 21 (21): 8412. doi:10.3390/ijms21218412. PMC 7665155. PMID 33182504.
- ^ Ness TL, Hogaboam CM, Kunkel SL (2006). "Chemokins, CC | TARC (CCL17)". Encyclopedia of Respiratory Medicine. Elsevier. pp. 380–385. doi:10.1016/b0-12-370879-6/00465-8. ISBN 978-0-12-370879-3.
- ^ Imai T, Yoshida T, Baba M, Nishimura M, Kakizaki M, Yoshie O (August 1996). "Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector". The Journal of Biological Chemistry. 271 (35): 21514–21521. doi:10.1074/jbc.271.35.21514. PMID 8702936.
- ^ Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O (June 1997). "The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4". The Journal of Biological Chemistry. 272 (23): 15036–15042. doi:10.1074/jbc.272.23.15036. PMID 9169480.
- Yoshie O, Matsushima K (January 2015). "CCR4 and its ligands: from bench to bedside". International Immunology. 27 (1): 11–20. doi:10.1093/intimm/dxu079. PMID 25087232.
- ^ Lee KM, Achuthan AA, Hamilton JA (October 2020). "GM-CSF: A Promising Target in Inflammation and Autoimmunity". ImmunoTargets and Therapy. 9: 225–240. doi:10.2147/itt.s262566. PMC 7605919. PMID 33150139.
- Dembic Z (2015). "Cytokines Important for Growth and/or Development of Cells of the Immune System". The Cytokines of the Immune System. Elsevier. pp. 263–281. ISBN 978-0-12-419998-9.
- van den Berg A, Visser L, Poppema S (June 1999). "High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin's lymphoma". The American Journal of Pathology. 154 (6): 1685–1691. doi:10.1016/S0002-9440(10)65424-7. PMC 1876772. PMID 10362793.
- Kilsdonk M, Veldman C, Rosati S, Plattel W, Diepstra A (February 2023). "The value of thymus and activation related chemokine immunohistochemistry in classic Hodgkin lymphoma diagnostics". Histopathology. 82 (3): 495–503. doi:10.1111/his.14836. PMC 10100154. PMID 36345263.
- Weniger MA, Küppers R (April 2021). "Molecular biology of Hodgkin lymphoma". Leukemia. 35 (4): 968–981. doi:10.1038/s41375-021-01204-6. PMC 8024192. PMID 33686198.
- Niens M, Visser L, Nolte IM, van der Steege G, Diepstra A, Cordano P, et al. (March 2008). "Serum chemokine levels in Hodgkin lymphoma patients: highly increased levels of CCL17 and CCL22". British Journal of Haematology. 140 (5): 527–536. doi:10.1111/j.1365-2141.2007.06964.x. PMID 18275430. S2CID 24343256.
- Plattel WJ, van den Berg A, Visser L, van der Graaf AM, Pruim J, Vos H, et al. (March 2012). "Plasma thymus and activation-regulated chemokine as an early response marker in classical Hodgkin's lymphoma". Haematologica. 97 (3): 410–415. doi:10.3324/haematol.2011.053199. PMC 3291596. PMID 22058214.
- Plattel WJ, Alsada ZN, van Imhoff GW, Diepstra A, van den Berg A, Visser L (December 2016). "Biomarkers for evaluation of treatment response in classical Hodgkin lymphoma: comparison of sGalectin-1, sCD163 and sCD30 with TARC". British Journal of Haematology. 175 (5): 868–875. doi:10.1111/bjh.14317. PMID 27610595. S2CID 23151893.
- Plattel WJ, Visser L, Diepstra A, Glaudemans AW, Nijland M, van Meerten T, et al. (July 2020). "Interim thymus and activation regulated chemokine versus interim F-fluorodeoxyglucose positron-emission tomography in classical Hodgkin lymphoma response evaluation". British Journal of Haematology. 190 (1): 40–44. doi:10.1111/bjh.16514. PMC 7383815. PMID 32106342.
- Driessen J, Kersten MJ, Visser L, van den Berg A, Tonino SH, Zijlstra JM, et al. (December 2022). "Prognostic value of TARC and quantitative PET parameters in relapsed or refractory Hodgkin lymphoma patients treated with brentuximab vedotin and DHAP" (PDF). Leukemia. 36 (12): 2853–2862. doi:10.1038/s41375-022-01717-8. PMID 36241696. S2CID 252904091.
- Viviani S, Mazzocchi A, Pavoni C, Taverna F, Rossi A, Patti C, et al. (October 2020). "Early serum TARC reduction predicts prognosis in advanced-stage Hodgkin lymphoma patients treated with a PET-adapted strategy". Hematological Oncology. 38 (4): 501–508. doi:10.1002/hon.2775. PMID 32602970. S2CID 220270572.
- Hsi ED, Li H, Nixon AB, Schöder H, Bartlett NL, LeBlanc M, et al. (April 2019). "Serum levels of TARC, MDC, IL-10, and soluble CD163 in Hodgkin lymphoma: a SWOG S0816 correlative study". Blood. 133 (16): 1762–1765. doi:10.1182/blood-2018-08-870915. PMC 6473498. PMID 30723079.
- Guidetti A, Mazzocchi A, Miceli R, Paterno' E, Taverna F, Spina F, et al. (November 2017). "Early reduction of serum TARC levels may predict for success of ABVD as frontline treatment in patients with Hodgkin Lymphoma". Leukemia Research. 62: 91–97. doi:10.1016/j.leukres.2017.09.018. PMID 28992524.
- Jones K, Vari F, Keane C, Crooks P, Nourse JP, Seymour LA, et al. (February 2013). "Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma". Clinical Cancer Research. 19 (3): 731–742. doi:10.1158/1078-0432.CCR-12-2693. PMID 23224400. S2CID 14252747.
- Diepstra A, Nolte IM, van den Berg A, Magpantay LI, Martínez-Maza O, Levin LI (November 2023). "Elevated serum TARC levels precede classic Hodgkin lymphoma diagnosis by several years". Blood. 142 (22): 1928–1931. doi:10.1182/blood.2023020959. PMC 10733822. PMID 37748137. S2CID 262751338.
- ^ Furue M (2018-07-25). "T helper type 2 signatures in atopic dermatitis". Journal of Cutaneous Immunology and Allergy. 1 (3): 93–99. doi:10.1002/cia2.12023. S2CID 91749090.
- ^ Catherine J, Roufosse F (June 2021). "What does elevated TARC/CCL17 expression tell us about eosinophilic disorders?". Seminars in Immunopathology. 43 (3): 439–458. doi:10.1007/s00281-021-00857-w. PMC 8132044. PMID 34009399.
- ^ Scheu S, Ali S, Ruland C, Arolt V, Alferink J (November 2017). "The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity". International Journal of Molecular Sciences. 18 (11): 2306. doi:10.3390/ijms18112306. PMC 5713275. PMID 29099057.
- Kataoka Y (March 2014). "Thymus and activation-regulated chemokine as a clinical biomarker in atopic dermatitis". The Journal of Dermatology. 41 (3): 221–229. doi:10.1111/1346-8138.12440. PMID 24628072. S2CID 7776726.
- Renert-Yuval Y, Thyssen JP, Bissonnette R, Bieber T, Kabashima K, Hijnen D, Guttman-Yassky E (April 2021). "Biomarkers in atopic dermatitis-a review on behalf of the International Eczema Council". The Journal of Allergy and Clinical Immunology. 147 (4): 1174–1190.e1. doi:10.1016/j.jaci.2021.01.013. hdl:1765/135258. PMC 11304440. PMID 33516871. S2CID 231756123.
- ^ Furue M, Ulzii D, Vu YH, Tsuji G, Kido-Nakahara M, Nakahara T (June 2019). "Pathogenesis of Atopic Dermatitis: Current Paradigm". Iranian Journal of Immunology. 16 (2): 97–107. doi:10.22034/iji.2019.80253. PMID 31182684.
- Kataoka Y (March 2014). "Thymus and activation-regulated chemokine as a clinical biomarker in atopic dermatitis". The Journal of Dermatology. 41 (3): 221–229. doi:10.1111/1346-8138.12440. PMID 24628072. S2CID 7776726.
- Renert-Yuval Y, Thyssen JP, Bissonnette R, Bieber T, Kabashima K, Hijnen D, Guttman-Yassky E (April 2021). "Biomarkers in atopic dermatitis-a review on behalf of the International Eczema Council". The Journal of Allergy and Clinical Immunology. 147 (4): 1174–1190.e1. doi:10.1016/j.jaci.2021.01.013. hdl:1765/135258. PMC 11304440. PMID 33516871. S2CID 231756123.
- ^ Kothur K, Wienholt L, Brilot F, Dale RC (January 2016). "CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review". Cytokine. 77: 227–237. doi:10.1016/j.cyto.2015.10.001. PMID 26463515.
- Chang KH, Ro LS, Lyu RK, Chen CM (February 2015). "Biomarkers for neuromyelitis optica". Clinica Chimica Acta; International Journal of Clinical Chemistry. 440: 64–71. doi:10.1016/j.cca.2014.11.004. PMID 25444748.
- Nomiyama H, Imai T, Kusuda J, Miura R, Callen DF, Yoshie O (February 1997). "Assignment of the human CC chemokine gene TARC (SCYA17) to chromosome 16q13". Genomics. 40 (1): 211–213. doi:10.1006/geno.1996.4552. PMID 9070951.
- Nomiyama H, Imai T, Kusuda J, Miura R, Callen DF, Yoshie O (1998). "Human chemokines fractalkine (SCYD1), MDC (SCYA22) and TARC (SCYA17) are clustered on chromosome 16q13". Cytogenetics and Cell Genetics. 81 (1): 10–11. doi:10.1159/000015000. PMID 9691168. S2CID 46851784.
Further reading
- Saeki H, Tamaki K (August 2006). "Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases". Journal of Dermatological Science. 43 (2): 75–84. doi:10.1016/j.jdermsci.2006.06.002. PMID 16859899.
- Imai T, Yoshida T, Baba M, Nishimura M, Kakizaki M, Yoshie O (August 1996). "Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector". The Journal of Biological Chemistry. 271 (35): 21514–21521. doi:10.1074/jbc.271.35.21514. PMID 8702936.
- Nomiyama H, Imai T, Kusuda J, Miura R, Callen DF, Yoshie O (February 1997). "Assignment of the human CC chemokine gene TARC (SCYA17) to chromosome 16q13". Genomics. 40 (1): 211–213. doi:10.1006/geno.1996.4552. PMID 9070951.
- Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O (June 1997). "The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4". The Journal of Biological Chemistry. 272 (23): 15036–15042. doi:10.1074/jbc.272.23.15036. PMID 9169480.
- Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, et al. (January 1998). "Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4". The Journal of Biological Chemistry. 273 (3): 1764–1768. doi:10.1074/jbc.273.3.1764. PMID 9430724.
- Bernardini G, Hedrick J, Sozzani S, Luini W, Spinetti G, Weiss M, et al. (February 1998). "Identification of the CC chemokines TARC and macrophage inflammatory protein-1 beta as novel functional ligands for the CCR8 receptor". European Journal of Immunology. 28 (2): 582–588. doi:10.1002/(SICI)1521-4141(199802)28:02<582::AID-IMMU582>3.0.CO;2-A. PMID 9521068.
- Nomiyama H, Imai T, Kusuda J, Miura R, Callen DF, Yoshie O (1998). "Human chemokines fractalkine (SCYD1), MDC (SCYA22) and TARC (SCYA17) are clustered on chromosome 16q13". Cytogenetics and Cell Genetics. 81 (1): 10–11. doi:10.1159/000015000. PMID 9691168. S2CID 46851784.
- Struyf S, Proost P, Sozzani S, Mantovani A, Wuyts A, De Clercq E, et al. (September 1998). "Enhanced anti-HIV-1 activity and altered chemotactic potency of NH2-terminally processed macrophage-derived chemokine (MDC) imply an additional MDC receptor". Journal of Immunology. 161 (6): 2672–2675. doi:10.4049/jimmunol.161.6.2672. PMID 9743322. S2CID 25336802.
- Loftus BJ, Kim UJ, Sneddon VP, Kalush F, Brandon R, Fuhrmann J, et al. (September 1999). "Genome duplications and other features in 12 Mb of DNA sequence from human chromosome 16p and 16q". Genomics. 60 (3): 295–308. doi:10.1006/geno.1999.5927. PMID 10493829.
- Garlisi CG, Xiao H, Tian F, Hedrick JA, Billah MM, Egan RW, Umland SP (October 1999). "The assignment of chemokine-chemokine receptor pairs: TARC and MIP-1 beta are not ligands for human CC-chemokine receptor 8". European Journal of Immunology. 29 (10): 3210–3215. doi:10.1002/(SICI)1521-4141(199910)29:10<3210::AID-IMMU3210>3.0.CO;2-W. PMID 10540332.
- Ghia P, Transidico P, Veiga JP, Schaniel C, Sallusto F, Matsushima K, et al. (August 2001). "Chemoattractants MDC and TARC are secreted by malignant B-cell precursors following CD40 ligation and support the migration of leukemia-specific T cells". Blood. 98 (3): 533–540. doi:10.1182/blood.V98.3.533. PMID 11468146.
- Morita A, Kikuoka S, Horikawa T, Bito T, Yamada H, Kanda M, et al. (August 2002). "Evaluation of human thymus and activation-regulated chemokine concentrations in blood using a new sandwich ELISA based on monoclonal antibodies". Clinica Chimica Acta; International Journal of Clinical Chemistry. 322 (1–2): 67–75. doi:10.1016/S0009-8981(02)00131-6. PMID 12104083.
- Basu S, Schaefer TM, Ghosh M, Fuller CL, Reinhart TA (May 2002). "Molecular cloning and sequencing of 25 different rhesus macaque chemokine cDNAs reveals evolutionary conservation among C, CC, CXC, AND CX3C families of chemokines". Cytokine. 18 (3): 140–148. doi:10.1006/cyto.2002.0875. PMID 12126650.
- D'Ambrosio D, Albanesi C, Lang R, Girolomoni G, Sinigaglia F, Laudanna C (September 2002). "Quantitative differences in chemokine receptor engagement generate diversity in integrin-dependent lymphocyte adhesion". Journal of Immunology. 169 (5): 2303–2312. doi:10.4049/jimmunol.169.5.2303. PMID 12193695.
- Matsumoto N, Mukae H, Nakamura-Uchiyama F, Ashitani JI, Abe K, Katoh S, et al. (November 2002). "Elevated levels of thymus and activation-regulated chemokine (TARC) in pleural effusion samples from patients infested with Paragonimus westermani". Clinical and Experimental Immunology. 130 (2): 314–318. doi:10.1046/j.1365-2249.2002.01985.x. PMC 1906524. PMID 12390321.
- Zheng X, Nakamura K, Tojo M, Oyama N, Nishibu A, Satoh M, et al. (November 2002). "TGF-beta1-mediated regulation of thymus and activation-regulated chemokine (TARC/CCL17) synthesis and secretion by HaCaT cells co-stimulated with TNF-alpha and IFN-gamma". Journal of Dermatological Science. 30 (2): 154–160. doi:10.1016/S0923-1811(02)00071-3. PMID 12413771.
- Kakinuma T, Nakamura K, Wakugawa M, Yano S, Saeki H, Torii H, et al. (October 2002). "IL-4, but not IL-13, modulates TARC (thymus and activation-regulated chemokine)/CCL17 and IP-10 (interferon-induced protein of 10kDA)/CXCL10 release by TNF-alpha and IFN-gamma in HaCaT cell line". Cytokine. 20 (1): 1–6. doi:10.1006/cyto.2002.1965. PMID 12441140.
- Uchida T, Suto H, Ra C, Ogawa H, Kobata T, Okumura K (December 2002). "Preferential expression of T(h)2-type chemokine and its receptor in atopic dermatitis". International Immunology. 14 (12): 1431–1438. doi:10.1093/intimm/dxf109. PMID 12456591.
- Jones K, Vari F, Keane C, Crooks P, Nourse JP, Seymour LA, et al. (February 2013). "Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma". Clinical Cancer Research. 19 (3): 731–742. doi:10.1158/1078-0432.CCR-12-2693. PMID 23224400.
External links
- Human CCL17 genome location and CCL17 gene details page in the UCSC Genome Browser.
| PDB gallery | |
|---|---|
| Cell signaling: cytokines | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| By family |
| ||||||||||||||||||||||||||||||||||||||||||
| By function/ cell | |||||||||||||||||||||||||||||||||||||||||||
| Chemokine receptor modulators | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC |
| ||||||||||||||||||||||||
| CXC |
| ||||||||||||||||||||||||
| C (XC) |
| ||||||||||||||||||||||||
| CX3C |
| ||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||