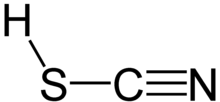
| |
 Carbon, C Sulfur, S Nitrogen, N Hydrogen, H | |
| Names | |
|---|---|
| IUPAC name Thiocyanic acid | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.672 |
| EC Number |
|
| Gmelin Reference | 25178 |
| KEGG | |
| MeSH | thiocyanic+acid |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | HSCN |
| Molar mass | 59.09 g·mol |
| Appearance |
|
| Odor | Pungent |
| Density | 2.04 g/cm |
| Melting point |
|
| Solubility in water | Miscible |
| Solubility | Soluble in ethanol, diethyl ether |
| log P | 0.429 |
| Vapor pressure | 4.73 mmHg (631 Pa) |
| Acidity (pKa) | 0.926 |
| Basicity (pKb) | 13.071 |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H312, H332, H412 |
| Precautionary statements | P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P501 |
| Related compounds | |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Thiocyanic acid is a chemical compound with the formula HSCN and structure H−S−C≡N, which exists as a tautomer with isothiocyanic acid (H−N=C=S). The isothiocyanic acid tautomer tends to dominate with the compound being about 95% isothiocyanic acid in the vapor phase.
It is a moderately strong acid, with a pKa of 1.1 at 20 °C and extrapolated to zero ionic strength.
One of the thiocyanic acid tautomers, HSCN, is predicted to have a triple bond between carbon and nitrogen. Thiocyanic acid has been observed spectroscopically.
The salts and esters of thiocyanic acid are known as thiocyanates. The salts are composed of the thiocyanate ion ([SCN]) and a suitable cation (e.g., potassium thiocyanate, KSCN). The esters of thiocyanic acid have the general structure R−S−C≡N, where R stands for an organyl group.
Isothiocyanic acid, HNCS, is a Lewis acid whose free energy, enthalpy and entropy changes for its 1:1 association with a variety of Lewis bases in carbon tetrachloride solution at 25 °C have been reported.< HNCS acceptor properties are discussed in the ECW model. The salts are composed of the thiocyanate ion ([SCN]) and a suitable cation (e.g., ammonium thiocyanate, [NH4][SCN]). Isothiocyanic acid forms isothiocyanates R−N=C=S, where R stands for an organyl group.
Thiocyanuric acid is a stable trimer of thiocyanic acid.
References
- Merck Index, 11th Edition, 9257.
- ^ "Thiocyanic acid". The Merck Index. Royal Society of Chemistry.
- von Richter, Victor (1922). Organic Chemistry or Chemistry of the Carbon Compounds. Vol. 1. Translated by Spielmann, Percy E. Philadelphia: P. Blakiston's Son & Co. p. 466.
- "Thiocyanic acid" entry in PubChem (database).
- ^ ILO and WHO staff. "Thiocyanic acid" safety card. European Commission
- Birckenbach, Lothar (1942). Forschungen und Fortschritte. 18: 232–3
{{cite journal}}: Missing or empty|title=(help). As cited in CAS Common Chemistry. - Brown, Jay A. (ed.; 2024), "Thiocyanic Acid" in Haz-Map (database). Engineered IT.
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- Beard, C. I.; Dailey, B. P. (1950). "The Structure and Dipole Moment of Isothiocyanic Acid" (PDF). The Journal of Chemical Physics. 18 (11): 1437. Bibcode:1950JChPh..18.1437B. doi:10.1063/1.1747507. hdl:1721.1/4934.
- Munegumi, Toratane (23 January 2013). "Where is the Border Line between Strong Acids and Weak Acids?". World Journal of Chemical Education. 1 (1): 12–16.
- Martell, A. E.; Smith, R. M.; Motelaitis, R. J. (2001). NIST Database 46. Gaithersburg, MD: National Institute of Standards and Technology.
- Wierzejewska, M.; Mielke, Z. (2001). "Photolysis of Isothiocyanic Acid HNCS in Low-Temperature Matrices. Infrared Detection of HSCN and HSNC Isomers". Chemical Physics Letters. 349 (3–4): 227–234. Bibcode:2001CPL...349..227W. doi:10.1016/S0009-2614(01)01180-0.
- Barakat, T. M.; Nelson, Jane; Nelson, S. M.; Pullin, A. D. E. (1969). "Spectra and hydrogen-bonding of characteristics of thiocyanic acid. Part 4.—Association with weak proton acceptors". Trans. Faraday Soc. 65: 41–51. doi:10.1039/tf9696500041. ISSN 0014-7672.
| Salts and covalent derivatives of the thiocyanate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen compounds | |
|---|---|
|
| Sulfur compounds | |||||
|---|---|---|---|---|---|
| Sulfides and disulfides |
| ||||
| Sulfur halides | |||||
| Sulfur oxides and oxyhalides |
| ||||
| Sulfur nitrides | |||||
| Thiocyanates | |||||
| Organic compounds | |||||

