| The topic of this article may not meet Misplaced Pages's general notability guideline. Please help to demonstrate the notability of the topic by citing reliable secondary sources that are independent of the topic and provide significant coverage of it beyond a mere trivial mention. If notability cannot be shown, the article is likely to be merged, redirected, or deleted. Find sources: "Chunganenol" – news · newspapers · books · scholar · JSTOR (August 2014) (Learn how and when to remove this message) |
| |
| Names | |
|---|---|
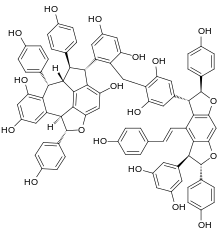
| Preferred IUPAC name (2S,2R,2R,2S,2S,2S,6S,6S,6S,6S)-2,2,6,6-Tetrakis(4-hydroxyphenyl)-6--2,2,2,2,2,2,6,6,6,6-decahydro-2(1,5)-benzoazulenobenzofurana-6(3,5)-benzodifurana-1,7(1),3(1,2),5(1,4)-tetrabenzenaheptaphane-1,2,2,2,3,3,5,5,7,7-decol | |
| Identifiers | |
| 3D model (JSmol) | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C85H64O18 |
| Molar mass | 1373.429 g·mol |
| Appearance | Colorless amorphous powder |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Chunganenol is a resveratrol hexamer found in Vitis chunganensis.
References
- Shan He; Liyan Jiang; Bin Wu; Chang Li; Yuanjiang Pan (2009). "Chunganenol: An Unusual Antioxidative Resveratrol Hexamer from Vitis chunganensis". J. Org. Chem. 74 (20): 7966–7969. doi:10.1021/jo901354p. PMID 19757796.
| Oligostilbenoids and their glycosides | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimers |
| ||||||||||||
| Trimers | |||||||||||||
| Tetramers: |
| ||||||||||||
| Higher polymers (five units or more) |
| ||||||||||||
| Oligomeric forms of resveratrol |
| ||||||||||||
| Glycosides or conjugates |
| ||||||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |