| This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this article by introducing citations to additional sources. Find sources: "Calcium iodate" – news · newspapers · books · scholar · JSTOR (November 2023) |
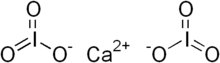
| |
| Names | |
|---|---|
| IUPAC name Calcium diiodate | |
| Other names Lautarite | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.265 |
| EC Number |
|
| E number | E916 (glazing agents, ...) |
| PubChem CID | |
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Ca(IO3)2 |
| Molar mass | 389.88 g/mol (anhydrous) 407.90 g/mol (monohydrate) |
| Appearance | white solid |
| Density | 4.519 g/cm (monohydrate) |
| Melting point | 540 °C (1,004 °F; 813 K) (monohydrate) |
| Boiling point | decomposes |
| Solubility in water | 0.09 g/100 mL (0 °C) 0.24 g/100 mL (20 °C) 0.67 g/100 mL (90 °C) |
| Solubility product (Ksp) | 6.47×10 |
| Solubility | soluble in nitric acid insoluble in alcohol |
| Magnetic susceptibility (χ) | -101.4·10 cm/mol |
| Structure | |
| Crystal structure | monoclinic (anhydrous) cubic (monohydrate) orthorhombic (hexahydrate) |
| Hazards | |
| Flash point | non-flammable |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Calcium iodate is any of two inorganic compounds with the formula Ca(IO3)2(H2O)x, where x = 0 or 1. Both are colourless salts that occur as the minerals lautarite and bruggenite, respectively. A third mineral form of calcium iodate is dietzeite, a salt containing chromate with the formula Ca2(IO3)2CrO4. These minerals are the most common compounds containing iodate.
Production and uses
Lautarite, described as the most important mineral source of iodine, is mined in the Atacama Desert. Processing of the ore entails reduction of its aqueous extracts with sodium bisulfite to give sodium iodide. This comproportionation reaction is a major source of the sodium iodide.
Calcium iodate can be produced by the anodic oxidation of calcium iodide or by passing chlorine into a hot solution of lime in which iodine has been dissolved.
Calcium iodate is used as an iodine supplement in chicken feed. Ethylenediamine dihydroiodide (EDDI) is a more typical source of nutritional iodine.
References
- ^ Lyday, Phyllis A.; Kaiho, Tatsuo (2015). "Iodine and Iodine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–13. doi:10.1002/14356007.a14_381.pub2. ISBN 978-3527306732.
| Calcium compounds | |
|---|---|
| Hydrogen & halogens | |
| Chalcogens | |
| Pnictogens | |
| Group 13 & 14 | |
| Trans metals | |
| Organics | |
| Iodates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||