| Revision as of 01:33, 8 December 2007 editDaveswenson (talk | contribs)3 editsNo edit summary← Previous edit | Latest revision as of 15:14, 14 December 2024 edit undoBeadbop (talk | contribs)Extended confirmed users699 editsm →Party use | ||
| Line 1: | Line 1: | ||
| {{short description|Chemical compound}} | |||
| <!--I removed the lowercase template; this is an article title and should be treated like the beginning of a sentence. --> | |||
| {{redirect|GHB}} | |||
| {{drugbox | | |||
| {{lowercase title}} | |||
| | image = 4-hydroxybutanoic-acid.png | |||
| {{Use dmy dates|date=March 2023}} | |||
| | image2= N2202291_35049282_595.jpg | |||
| {{cs1 config |name-list-style=vanc |display-authors=6}} | |||
| | width = 200 | |||
| {{Infobox drug | |||
| | imagename = gamma-Hydroxybutyric acid | |||
| | Watchedfields = changed | |||
| | IUPAC_name = 4-Hydroxybutanoic acid | |||
| | verifiedrevid = 477230013 | |||
| | CAS_number = 591-81-1 | |||
| | drug_name = γ-Hydroxybutyric acid | |||
| | ATC_prefix = N01 | |||
| | INN = | |||
| | ATC_suffix = AX11 | |||
| | type = <!-- empty --> | |||
| | PubChem = 3037032 | |||
| | image = 4-Hydroxybutansäure - 4-Hydroxybutanoic acid.svg | |||
| | DrugBank = ? | |||
| | |
| alt = | ||
| | image2 = GHB-3D-balls.png | |||
| | molecular_weight = 104.10 g/mol (GHB)<br>126.09 g/mol (sodium salt)<br>142.19 g/mol (potassium salt) | |||
| | alt2 = | |||
| | SMILES = OCCCC(=O)O | |||
| | caption = <!-- Clinical data --> | |||
| | bioavailability = 25% (oral) | |||
| | pronounce = | |||
| | metabolism = 95%, mainly ], also in blood and tissues | |||
| | tradename = | |||
| | elimination_half-life = 30 - 60 minutes | |||
| | Drugs.com = | |||
| | excretion = 5%, ] | |||
| | |
| MedlinePlus = | ||
| | licence_EU = <!-- EMA uses INN (or special INN_EMA) --> | |||
| | legal_AU = S9 | |||
| | DailyMedID = <!-- DailyMed may use generic or brand name (generic name preferred) --> | |||
| | legal_CA = Schedule III | |||
| | licence_US = <!-- FDA may use generic or brand name (generic name preferred) --> | |||
| | legal_US = Schedule I | |||
| | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| | legal_UK = Class C | |||
| | pregnancy_AU_comment = | |||
| | legal_status = Class B (]) | |||
| | pregnancy_category = | |||
| | routes_of_administration = Usually oral; ] | |||
| | dependency_liability = | |||
| }} | |||
| | addiction_liability = High<ref>Tay, E., Lo, W. K. W., & Murnion, B. (2022). Current Insights on the Impact of Gamma-Hydroxybutyrate (GHB) Abuse. Substance Abuse and Rehabilitation, 13, 13–23. {{doi|10.2147/SAR.S315720}}</ref> | |||
| | routes_of_administration = ], ] | |||
| | class = ], ]—]; ]; | |||
| ];<br> ]<br>] | |||
| | ATCvet = | |||
| | ATC_prefix = N01 | |||
| | ATC_suffix = AX11 | |||
| | ATC_supplemental = {{ATC|N07|XX04}} | |||
| <!-- Legal status -->| legal_AU = S9 | |||
| '''Gamma-Hydroxybutyric acid''' (4-hydroxybutanoic acid, C<sub>4</sub>H<sub>8</sub>O<sub>3</sub>), commonly abbreviated '''GHB''', is a ] ] that is ] in a number of countries<ref name="erowidGHBlaw">http://erowid.org/chemicals/ghb/ghb_law.shtml</ref>, and is a naturally-occurring substance found in the ], wine, beef, small citrus fruits, and almost all living creatures in small amounts. It is currently regulated in the US and sold by ] under the name ].<ref>http://stocks.us.reuters.com/stocks/fullDescription.asp?rpc=66&symbol=JAZZ.O</ref> | |||
| | legal_AU_comment = / S8 as ]<ref>{{cite web | url=https://www.legislation.gov.au/F2024L00589/latest/text | title=Therapeutic Goods (Poisons Standard—June 2024) Instrument 2024 | date=30 May 2024 }}</ref> | |||
| | legal_BR = B1 | |||
| | legal_BR_comment = <ref>{{Cite web |author=Anvisa |author-link=Brazilian Health Regulatory Agency |date=2023-03-31 |title=RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial |trans-title=Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control|url=https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992 |url-status=live |archive-url=https://web.archive.org/web/20230803143925/https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992 |archive-date=2023-08-03 |access-date=2023-08-16 |publisher=] |language=pt-BR |publication-date=2023-04-04}}</ref> | |||
| | legal_CA = Schedule I | |||
| | legal_CA_comment = | |||
| | legal_DE = Rx-only/Anlage III | |||
| | legal_DE_comment = | |||
| | legal_NZ = Class B | |||
| | legal_NZ_comment = | |||
| | legal_UK = Class B | |||
| | legal_UK_comment = | |||
| | legal_US = Schedule I | |||
| | legal_US_comment = / Schedule III (] and ])<ref>{{cite web|url=https://www.dea.gov/sites/default/files/2020-06/GHB-2020_0.pdf |archive-url=https://web.archive.org/web/20201017155504/https://www.dea.gov/sites/default/files/2020-06/GHB-2020_0.pdf |archive-date=17 October 2020 |url-status=live|title=What is GHB?|website=Dea.gov|access-date=6 March 2022}}</ref><ref name="DEA" /> | |||
| | legal_EU = ] | |||
| | legal_EU_comment = (]) | |||
| | legal_UN = P II | |||
| | legal_UN_comment = | |||
| | legal_status = <!-- For countries not listed above --> | |||
| <!-- Pharmacokinetic data -->| bioavailability = 25% (oral) | |||
| In a medical setting, GHB has been used historically as a general anesthetic, to treat conditions such as insomnia, clinical depression, narcolepsy, and alcoholism, and to improve athletic performance<ref name="emedicineonGHB">{{cite web | title = Toxicity, Gamma-Hydroxybutyrate | date = ], ] | author = Theodore I Benzer | url = http://www.emedicine.com/emerg/topic848.htm | publisher = ]}}</ref>. It is also used ] under the street names ''Juice'', ''Liquid Ecstasy'', ''Fantasy'', "Georgia Homeboy", and simply ''G'', either as an intoxicant or as a ]. GHB is naturally produced in the human body's cells and is structurally related to the ] ]. As a drug, it is used most commonly in the form of a ]. <ref>e.g., '''sodium gamma-hydroxybutyrate''' (Na.GHB, '''sodium oxybate''') or potassium gamma-hydroxybutyrate (K.GHB)</ref> GHB is also produced as a result of fermentation, and so is found in small quantities in some beers and wines. | |||
| | protein_bound = | |||
| | metabolism = 95–98%, mainly ], also in blood and tissues<ref name="auto" /> | |||
| | metabolites = | |||
| | onset = Within 5–15 minutes<ref>{{cite book| vauthors = Riviello RJ |title=Manual of forensic emergency medicine : a guide for clinicians|date=2010|publisher=Jones and Bartlett Publishers|location=Sudbury, MA|isbn=978-0763744625|page=42|url=https://books.google.com/books?id=keng9ELAE2IC&pg=PA42}}</ref> | |||
| | elimination_half-life = 30–60 minutes | |||
| | duration_of_action = | |||
| | excretion = 1–5%, ]<ref name="auto" /> | |||
| <!-- Identifiers -->| CAS_number_Ref = {{cascite|correct|??}} | |||
| == History == | |||
| | CAS_number = 591-81-1 | |||
| Synthesis of the chemical GHB was first reported in 1874 by A. Saizew,<ref> 1874 Saizew,A. JLACBF Justus Liebigs Ann. Chem. 171 274. </ref> but the first major research into its use in humans was conducted in the early 1960s by Dr. ] to use in studying the neurotransmitter ].{{Fact|date=February 2007}} It quickly found a wide range of uses due to its minimal side-effects and short duration of action, the only difficulties being the narrow safe dosage range and the dangers presented by its combination with ] and other CNS depressants. | |||
| | CAS_supplemental = | |||
| | PubChem = 3037032 | |||
| | IUPHAR_ligand = 4711 | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | DrugBank = DB01440 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 9984 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 30IW36W5B2 | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | KEGG = C00989 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 30830 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 1342 | |||
| | NIAID_ChemDB = | |||
| | PDB_ligand = | |||
| | synonyms = {{ubl|γ-hydroxybutyric acid|γ-hydroxybutyrate|GHB|fishies<ref>{{cite web|url=https://theconversation.com/pingers-pingas-pingaz-how-drug-slang-affects-the-way-we-use-and-understand-drugs-129452|title=Pingers, pingas, pingaz: how drug slang affects the way we use and understand drugs|publisher=]|date=8 January 2020|archive-url=https://web.archive.org/web/20210115205246/https://theconversation.com/pingers-pingas-pingaz-how-drug-slang-affects-the-way-we-use-and-understand-drugs-129452|archive-date=2021-01-15|url-status=live|access-date=13 May 2023}}</ref>|G<ref>{{Cite web |url=https://www.letstalkaboutit.nhs.uk/directory-of-services/chemsex-support/ghb-gbl-g/ |title=GHB/GBL "G" |access-date=24 May 2024 |archive-date=24 May 2024 |archive-url=https://web.archive.org/web/20240524154840/https://www.letstalkaboutit.nhs.uk/directory-of-services/chemsex-support/ghb-gbl-g/ |url-status=live }}</ref>}} | |||
| <!-- Chemical and physical data -->| IUPAC_name = 4-hydroxybutanoic acid | |||
| GHB was widely used in France, Italy, and other European countries for several decades as a sleeping agent and an anaesthetic in childbirth, but problems with its abuse potential and development of newer drugs have led to a decrease in legitimate medical use of GHB in recent times. The only common medical applications for GHB now days are in the treatment of ] and more rarely alcoholism. In the typical scenario, GHB has been synthesized from GBL (]) by adding ] (lye) in ethanol or water. As of late, GBL has become controlled and more circuitous routes have to be taken, such as those starting with THF (]). | |||
| | C = 4 | |||
| | H = 8 | |||
| | O = 3 | |||
| | SMILES = O=C(O)CCCO | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C4H8O3/c5-3-1-2-4(6)7/h5H,1-3H2,(H,6,7) | |||
| | StdInChI_comment = | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = SJZRECIVHVDYJC-UHFFFAOYSA-N | |||
| | density = | |||
| | density_notes = | |||
| | melting_point = | |||
| | melting_high = | |||
| | melting_notes = | |||
| | boiling_point = | |||
| | boiling_notes = | |||
| | solubility = | |||
| | sol_units = | |||
| | specific_rotation = | |||
| }} | |||
| '''γ-Hydroxybutyric acid''', also known as '''''gamma''-hydroxybutyric acid''', '''GHB''', or '''4-hydroxybutanoic acid''', is a naturally occurring ] and a ]. It is a precursor to ], ], and ] in certain brain areas. It acts on the ] and is a weak ] at the ] receptor. GHB has been used in the medical setting as a ] and as treatment for ], ], and ].<ref>{{cite web |url=https://www.nlm.nih.gov/medlineplus/druginfo/meds/a605032.html |title=Sodium Oxybate: MedlinePlus Drug Information |publisher=Nlm.nih.gov |date=28 July 2010 |access-date=1 August 2010 |archive-date=11 April 2010 |archive-url=https://web.archive.org/web/20100411105041/http://www.nlm.nih.gov/medlineplus/druginfo/meds/a605032.html |url-status=live }}</ref><ref name="emedicineonGHB">{{cite web | vauthors = Benzer TI, Cameron S | veditors = VanDeVoort JT, Benitez JG | title = Toxicity, Gamma-Hydroxybutyrate | date = 8 January 2007 | url = http://www.emedicine.com/emerg/topic848.htm | publisher = ] | access-date = 16 January 2007 | archive-date = 28 November 2021 | archive-url = https://web.archive.org/web/20211128091203/http://emedicine.medscape.com/article/820531-overview | url-status = live }}</ref> The substance is also used illicitly for various reasons, including as a ], ], and as a ].<ref name="dea-daterape" /> | |||
| It is commonly used in the form of a salt, such as sodium γ-hydroxybutyrate (NaGHB, ], or Xyrem) or potassium γ-hydroxybutyrate (KGHB, potassium oxybate). GHB is also produced as a result of fermentation, and is found in small quantities in some beers and wines, beef, and small citrus fruits.<ref name="Choc_to_Morph">{{cite book | vauthors = Weil A, Winifred R |author-link= Andrew Weil |title= From Chocolate to Morphine |edition= 2nd |year= 1993 |publisher= Houghton Mifflin Company |location= Boston/New York |isbn= 978-0-395-66079-9 |page= |chapter= Depressants |chapter-url-access= registration |chapter-url= https://archive.org/details/fromchocolatetom00weil |url= https://archive.org/details/fromchocolatetom00weil/page/77 }}</ref> | |||
| A popular children's toy, ] (also known as Aqua Dots, in the United States), produced by Melbourne company Moose, was banned in Australia in early November 2007 when it was discovered that ], which is ] into GHB, had been substituted for the non-toxic plasticiser ] in the bead manufacturing process. Three young children were hospitalized as a result of ingesting a large number of the beads, and the toy was recalled.<ref>{{Cite news | |||
| | author = Michael Perry, James Pomfret, Roger Crabb | |||
| | title = Australia bans China-made toy on toxic drug risk | |||
| | date = Nov 7, 2007 | |||
| | publisher = ] | |||
| | url = http://www.reuters.com/article/worldNews/idUSSYD2129620071107 | |||
| }}</ref> | |||
| ] is a disease that causes GHB to accumulate in the blood. | |||
| == Pharmacology == | |||
| GHB has at least two distinct binding sites<ref>http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=15567424&ordinalpos=25&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref> in the ], the newly-characterized ], which is ], and the ] receptor, which is ]. GHB exists endogenously at concentrations high enough to activate the GHB receptor, but not at concentrations high enough to activate the GABA<sub>B</sub> receptor. This means that endogenous GHB has all the characteristics of an ] ]. | |||
| ==Medical use== | |||
| When taken orally, GABA itself can not cross the Blood-Brain-Barrier nor does a high concentration actually reach the GABA receptors once in the brain. Since GABA is naturally systhesized in the brain, a higher than normal concentration will be quickly neutralized. <ref>http://www.bio.net/bionet/mm/neur-sci/1999-May/038337.html</ref> | |||
| {{main|Sodium oxybate}} | |||
| GHB is used for medical purposes in the treatment of ]<ref>{{cite journal |vauthors = Mayer G |title = The use of sodium oxybate to treat narcolepsy |journal = Expert Review of Neurotherapeutics |volume = 12 |issue = 5 |pages = 519–29 |date = May 2012 |pmid = 22550980 |doi = 10.1586/ern.12.42 |s2cid = 43706704 }}</ref> and, more rarely, ],<ref>{{cite journal |vauthors = Caputo F, Mirijello A, Cibin M, Mosti A, Ceccanti M, Domenicali M, Bernardi M, Maremmani I, Addolorato G |title = Novel strategies to treat alcohol dependence with sodium oxybate according to clinical practice |journal = European Review for Medical and Pharmacological Sciences |volume = 19 |issue = 7 |pages = 1315–20 |date = April 2015 |pmid = 25912595 }}</ref><ref>{{cite journal |vauthors = Keating GM |title = Sodium oxybate: a review of its use in alcohol withdrawal syndrome and in the maintenance of abstinence in alcohol dependence | journal = Clinical Drug Investigation |volume = 34 |issue = 1 |pages = 63–80 | date = January 2014 |pmid = 24307430 |doi = 10.1007/s40261-013-0158-x |s2cid = 2056246}}</ref> although there remains uncertainty about its efficacy relative to other pharmacotherapies for alcohol dependence.<ref>{{cite journal |vauthors = Leone MA, Vigna-Taglianti F, Avanzi G, Brambilla R, Faggiano F |title = Gamma-hydroxybutyrate (GHB) for treatment of alcohol withdrawal and prevention of relapses |journal = The Cochrane Database of Systematic Reviews |issue = 2 | pages = CD006266 |date = February 2010 |pmid = 20166080 |doi = 10.1002/14651858.CD006266.pub2 |collaboration = Cochrane Drugs and Alcohol Group |quote= There is insufficient randomised evidence to be confident of a difference between GHB and placebo, or to determine reliably if GHB is more or less effective than other drugs for the treatment of alcohol {{sic|?|withdrawl}} or the prevention of relapses.}}</ref> The authors of a 2010 Cochrane review<ref>{{Cite journal |url=https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006266.pub2/full |title=cochranelibrary.com |date=2010 |doi=10.1002/14651858.CD006266.pub2 |pmid=20166080 |access-date=23 January 2023 |archive-date=5 November 2022 |archive-url=https://web.archive.org/web/20221105110933/https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006266.pub2/full |url-status=live |journal=The Cochrane Database of Systematic Reviews |issue=2 |pages=CD006266 | vauthors = Leone MA, Vigna-Taglianti F, Avanzi G, Brambilla R, Faggiano F }}</ref> concluded that "GHB appears better than NTX and ] in maintaining abstinence and preventing craving in the medium term (3 to 12 months)". It is sometimes used ] for the treatment of ].<ref>{{cite journal |vauthors = Calandre EP, Rico-Villademoros F, Slim M |title = An update on pharmacotherapy for the treatment of fibromyalgia |journal = Expert Opinion on Pharmacotherapy |volume = 16 |issue = 9 |pages = 1347–68 |date = June 2015 |pmid = 26001183 |doi=10.1517/14656566.2015.1047343 |s2cid = 24246355}}</ref><ref>{{cite journal |vauthors = Staud R |title = Sodium oxybate for the treatment of fibromyalgia |journal = Expert Opinion on Pharmacotherapy |volume = 12 |issue = 11 |pages = 1789–98 |date = August 2011 |pmid = 21679091 |doi=10.1517/14656566.2011.589836 |s2cid = 33026097 }}</ref> GHB is the active ingredient of the ] ] (Xyrem). Sodium oxybate is approved by the ] for the treatment of cataplexy associated with narcolepsy<ref>{{Cite web |url=http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2002/21196ltr.pdf |archive-url=https://web.archive.org/web/20121017094123/http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2002/21196ltr.pdf |archive-date=17 October 2012 |url-status=live |title=FDA Approval Letter for Xyrem; Indication: Cataplexy associated with narcolepsy; 17 July 2002 |access-date=6 March 2022}}</ref> and ] (EDS) associated with narcolepsy.<ref>{{Cite web |url=http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2005/021196s005ltr.pdf |archive-url=https://web.archive.org/web/20121017094134/http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2005/021196s005ltr.pdf |archive-date=17 October 2012 |url-status=live |title=FDA Approval Letter for Xyrem; Indication: EDS (Excessive Daytime Sleepiness) associated with narcolepsy; 18 November 2005 |access-date=6 March 2022}}</ref> | |||
| GHB has been shown to reliably increase ]<ref name="pmid2281247">{{cite journal |vauthors = Scrima L, Hartman PG, Johnson FH, Thomas EE, Hiller FC |title = The effects of gamma-hydroxybutyrate on the sleep of narcolepsy patients: a double-blind study |journal = Sleep |volume = 13 |issue = 6 |pages = 479–90 |date = December 1990 |pmid = 2281247 |doi = 10.1093/sleep/13.6.479 |doi-access = free }}</ref><ref>{{cite journal |vauthors = Scrima L, Johnson FH, Hiller FC |title = Long-Term Effect of Gamma-Hydroxybutyrate on Sleep in Narcolepsy Patients |journal=Sleep Research |year=1991 |volume=20 |pages=330}}</ref><ref name="pmid9239423">{{cite journal |vauthors = Van Cauter E, Plat L, Scharf MB, Leproult R, Cespedes S, L'Hermite-Balériaux M, Copinschi G |title = Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men |journal = The Journal of Clinical Investigation |volume = 100 |issue = 3 |pages = 745–53 |date = August 1997 |pmid = 9239423 |pmc = 508244 |doi = 10.1172/JCI119587 }}</ref> and decrease the tendency for REM sleep in modified multiple sleep latency tests.<ref>{{cite journal |vauthors = Scrima L, Shander D |title = Letter to Editor on article: Re: Narcolepsy Review (Aldrich MS: 8-9-91) |journal = The New England Journal of Medicine |volume = 324 |issue = 4 |pages = 270–72 |date = January 1991 |pmid = 1985252 |doi = 10.1056/nejm199101243240416 }}</ref><ref name="pmid2281247"/> | |||
| However, at pharmacological doses, GHB reaches much higher concentrations in the brain and activates GABA<sub>B</sub> receptors, which is responsible for its sedative effects.<ref>http://www.ncbi.nlm.nih.gov/sites/entrez?db=pubmed&uid=16129424&cmd=showdetailview</ref> GHB's sedative effects are blocked by GABA<sub>B</sub> antagonists. | |||
| The FDA-approved labeling for sodium oxybate<ref>{{cite web |title = FDA Approved Labeling Text: Xyrem® (sodium oxybate) oral solution |date = 18 November 2005 |url = https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021196s005lbl.pdf |publisher = U.S. Food and Drug Administration |access-date = 23 April 2021 |archive-date = 24 January 2022 |archive-url = https://web.archive.org/web/20220124165753/https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021196s005lbl.pdf |url-status = live }}</ref> suggests no evidence GHB has ], ] or hepatotoxic properties. Its favorable safety profile relative to ethanol may explain why GHB continues to be investigated as a candidate for ] substitution.<ref name="pmid34237655">{{cite journal |vauthors = Guiraud J, Addolorato G, Aubin HJ, Batel P, de Bejczy A, Caputo F, Goudriaan AE, Gual A, Lesch O, Maremmani I, Perney P, Poulnais R, Raffaillac Q, Soderpalm B, Spanagel R, Walter H, van den Brink W |title = Treating alcohol dependence with an abuse and misuse deterrent formulation of sodium oxybate: Results of a randomised, double-blind, placebo-controlled study |journal = European Neuropsychopharmacology |volume = 52 |issue = |pages = 18–30 |date = July 2021 |pmid = 34237655 |doi=10.1016/j.euroneuro.2021.06.003 |doi-access = free|hdl = 11392/2483205 |hdl-access = free }}</ref> | |||
| The role of the GHB receptor in the behavioural effects induced by GHB is more complex. GHB receptors are densely expressed in many areas of the brain, including the cortex and hippocampus, and these are the receptors that GHB displays the highest affinity for. There has been somewhat limited research into the GHB receptor - however, there is evidence that activation of the GHB receptor in some brain areas results in the release of ] - the principle excitatory neurotransmitter <ref>http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=14535954&ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref>. | |||
| Drugs which selectively activate the GHB receptor receptor cause absent seizures in high doses, as do GHB and GABA(B) agonists. <ref>http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=7791129&ordinalpos=5&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref>. | |||
| ==Recreational use== | |||
| Activation of both the GHB receptor and GABA(B) is responsible for the addictive profile of GHB. GHB's effect on dopamine release is biphasic<ref>http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=1847191&ordinalpos=4&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref> , low concentrations stimulate dopamine release via the GHB receptor. <ref>http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=2173754&ordinalpos=9&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref> Higher concentrations inhibit dopamine release - via GABA(B) receptors (as to other GABA(B) agonists - ]<ref>http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=8549640&ordinalpos=15&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref>, ]) - after the initial phase of inhibition, dopamine release is then increased via the GHB receptor. Both the inhibition and increase of dopamine release by GHB are inhibited by opioid antagonists (], ]). Dynorphin may play a role in the inhibition of dopamine release via ]<ref>http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=2691926&ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref>. | |||
| <!-- Do not add street names below without references that they are in common use. Otherwise they will be removed. Misplaced Pages is not a dictionary, a jargon guide, or a collection of local trivia. --> | |||
| GHB is a central nervous system ] used as an ].<ref name="schep">{{cite journal | vauthors = Schep LJ, Knudsen K, Slaughter RJ, Vale JA, Mégarbane B | title = The clinical toxicology of γ-hydroxybutyrate, γ-butyrolactone and 1,4-butanediol | journal = Clinical Toxicology | volume = 50 | issue = 6 | pages = 458–70 | date = July 2012 | pmid = 22746383 | doi = 10.3109/15563650.2012.702218 | s2cid = 19697449 }}</ref> It has many street names. Its effects have been described as comparable with ] (alcohol) and ] use, such as ], disinhibition, enhanced libido and ] states. A review comparing ethanol to GHB concluded that the dangers of the two drugs were similar.<ref>{{cite journal | vauthors = Sellman JD, Robinson GM, Beasley R | title = Should ethanol be scheduled as a drug of high risk to public health? | journal = Journal of Psychopharmacology | volume = 23 | issue = 1 | pages = 94–100 | date = January 2009 | pmid = 18583435 | doi = 10.1177/0269881108091596 }}</ref> At higher doses, GHB may induce ], ], ], ], visual disturbances, depressed ]ing, ], ], and death. One potential cause of death from GHB consumption is polydrug toxicity. Co-administration with other CNS depressants such as alcohol or ]s can result in an additive effect (potentiation), as they all bind to ] (or "GABA") receptor sites. The effects of GHB can last from 1.5 to 4 hours, or longer if large doses have been consumed.<ref name="GALL1">{{cite journal | vauthors = Galloway GP, Frederick-Osborne SL, Seymour R, Contini SE, Smith DE | title = Abuse and therapeutic potential of gamma-hydroxybutyric acid | journal = Alcohol | volume = 20 | issue = 3 | pages = 263–69 | date = April 2000 | pmid = 10869868 | doi = 10.1016/S0741-8329(99)00090-7 }}</ref> Consuming GHB with alcohol can cause respiratory arrest and vomiting in combination with unarousable sleep, which can lead to death.<ref>{{cite journal | vauthors = Thai D, Dyer JE, Benowitz NL, Haller CA | title = Gamma-hydroxybutyrate and ethanol effects and interactions in humans | journal = Journal of Clinical Psychopharmacology | volume = 26 | issue = 5 | pages = 524–29 | date = October 2006 | pmid = 16974199 | pmc = 2766839 | doi = 10.1097/01.jcp.0000237944.57893.28 }}</ref><ref> {{Webarchive|url=https://web.archive.org/web/20151122183546/https://www.erowid.org/chemicals/ghb/ghb_health.shtml |date=22 November 2015 }}. Erowid.org (18 March 2009). Retrieved on 27 September 2012.</ref> | |||
| Recreational doses of 1–2 g generally provide a feeling of euphoria, and larger doses create deleterious effects such as reduced motor function and drowsiness.<ref name="auto">{{cite journal | vauthors = Busardò FP, Jones AW | title = GHB pharmacology and toxicology: acute intoxication, concentrations in blood and urine in forensic cases and treatment of the withdrawal syndrome | journal = Current Neuropharmacology | volume = 13 | issue = 1 | pages = 47–70 | date = January 2015 | pmid = 26074743 | pmc = 4462042 | doi = 10.2174/1570159X13666141210215423 }}</ref> The sodium salt of GHB has a salty taste.<ref name=GALL1/> Other salt forms such as calcium GHB and magnesium GHB have also been reported,<ref>{{cite patent |country= US |number= 4393236 |title= Production of nonhygroscopic salts of 4-hydroxybutyric acid |inventor= Klosa, Joseph |gdate= 12 July 1983 }}</ref> but the sodium salt is by far the most common. | |||
| This explains the paradoxical mix of sedative and stimulatory properties of GHB, as well as the so-called "rebound" effect, experienced by individuals using GHB as a sleeping agent, where they awake suddenly after several hours of GHB-induced deep sleep. That is to say, that over time, the concentration of GHB in the system decreases below the threshold for significant GABA<sub>B</sub> receptor activation and activates predominantly the GHB receptor, leading to wakefulness. | |||
| Some ]s, such as ] (GBL), convert to GHB in the stomach and bloodstream. Other prodrugs exist, such as ] (1,4-B).<ref>{{Cite web|work=PubChem|title=1,4-Butanediol|url=https://pubchem.ncbi.nlm.nih.gov/compound/8064|access-date=15 January 2021|publisher=U.S National Library of Medicine|archive-date=1 February 2021|archive-url=https://web.archive.org/web/20210201111223/https://pubchem.ncbi.nlm.nih.gov/compound/8064|url-status=live}}</ref> GBL and 1,4-B are normally found as pure liquids, but they can be mixed with other more harmful solvents when intended for industrial use (e.g. as ] or varnish thinner).{{cn|date=February 2024}} | |||
| Recently, analogs of GHB, such as ] have been synthesised and tested on animals, in order to gain a better understanding of GHB's mode of action.<ref>http://jpet.aspetjournals.org/cgi/content/full/305/2/675</ref> Analogues of GHB such as 3-methyl-GHB, 4-methyl-GHB and 4-phenyl-GHB have been shown to produce similar effects to GHB in some animal studies, but these compounds are even less well researched than GHB itself. Of these analogues, only 4-methyl-GHB (gamma-hydroxyvaleric acid, GHV) and its prodrug form ] (GVL) have been reported as drugs of abuse in humans, and on the available evidence seem to be less potent but more toxic than GHB, with a particular tendency to cause nausea and vomiting. | |||
| GHB can be manufactured with little knowledge of chemistry, as it involves the mixing of its two precursors, GBL and an ] such as ], to form the GHB salt. Due to the ease of manufacture and the availability of its precursors, it is not usually produced in illicit laboratories like other synthetic drugs, but in private homes by low-level producers.<ref>{{cite journal | vauthors = Kapoor P, Deshmukh R, Kukreja I | title = GHB acid: A rage or reprive | journal = Journal of Advanced Pharmaceutical Technology & Research | volume = 4 | issue = 4 | pages = 173–178 | date = October 2013 | pmid = 24350046 | pmc = 3853692 | doi = 10.4103/2231-4040.121410 | doi-access = free }}</ref> | |||
| Other prodrug ester forms of GHB have also rarely been encountered by law enforcement, including 1,4-diacetoxybutane, methyl-4-acetoxybutanoate and ethyl-4-acetoxybutanoate, but these are generally covered by analogue laws in jurisdictions where GHB is illegal, and little is known about them beyond their presumably delayed onset and longer duration of action. The intermediate compound 4-hydroxybutaldehyde is also a prodrug for GHB, however as with all aldehydes this compound is caustic and is strong-smelling and foul-tasting; actual use of this compound as an intoxicant is likely to be unpleasant and result in severe nausea and vomiting. | |||
| GHB is colourless and odourless.<ref name="jones">{{cite journal | vauthors = Jones C | title = Suspicious death related to gamma-hydroxybutyrate (GHB) toxicity | journal = Journal of Clinical Forensic Medicine | volume = 8 | issue = 2 | pages = 74–76 | date = June 2001 | pmid = 15274975 | doi = 10.1054/jcfm.2001.0473 }}</ref> | |||
| ] | |||
| === Party use === | |||
| Also note that both of the metabolic breakdown pathways shown for GHB can run in either direction, depending on the concentrations of the substances involved, so the body can make its own GHB either from GABA or from succinic semialdehyde. Under normal physiological conditions, the concentration of GHB in the body is rather low, and the pathways would run in the reverse direction to what is shown here to produce endogenous GHB. However, when GHB is consumed for medical or recreational purposes its concentration in the body is much higher than normal, which changes the enzyme kinetics so that these pathways operate to metabolise GHB rather than producing it. | |||
| GHB has been used as a ], apparently starting in the 1990s, as small doses of GHB can act as a euphoriant and are believed to be aphrodisiac.<ref>{{cite journal | vauthors = Kam PC, Yoong FF | title = Gamma-hydroxybutyric acid: an emerging recreational drug | journal = Anaesthesia | volume = 53 | issue = 12 | pages = 1195–98 | date = December 1998 | pmid = 10193223 | doi = 10.1046/j.1365-2044.1998.00603.x | doi-access=free | quote = In the UK, GHB has been available in the night clubs around London since 1994... }}</ref><ref name="Carter2009">{{cite journal | vauthors = Carter LP, Pardi D, Gorsline J, Griffiths RR | title = Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse | journal = Drug and Alcohol Dependence | volume = 104 | issue = 1–2 | pages = 1–10 | date = September 2009 | pmid = 19493637 | pmc = 2713368 | doi = 10.1016/j.drugalcdep.2009.04.012 }}</ref> Slang terms for GHB include ''liquid ecstasy'', ''lollipops'', ''liquid X'' or ''liquid E'' due to its tendency to produce euphoria and sociability and its use in the dance party scene.<ref>{{cite journal | vauthors = Klein M, Kramer F | title = Rave drugs: pharmacological considerations | journal = AANA Journal | volume = 72 | issue = 1 | pages = 61–67 | date = February 2004 | pmid = 15098519 }}</ref> | |||
| ===Sports and athletics=== | |||
| == Medical uses == | |||
| Some athletes have used GHB or its analogs because of being marketed as anabolic agents, although there is no evidence that it builds muscle or improves performance.<ref name=GALL1/> | |||
| GHB has been used historically as a general ] in the 1960s,<ref name="emedicineonGHB"/> as a ] in the treatment of ], to treat ], and to improve athletic performance. In Italy, under the trade name Alcover (ATC code N07BB), GHB is used in the treatment of ] (50 to 100 milligrams per kilogram per day, in 3 or more divided doses), both for acute alcohol withdrawal and medium to long-term detoxification.{{Fact|date=February 2007}} <ref>An author/scientist Gian Luigi Gessa has been researching alcoholism and the effects of various drugs to persons afflicted with said disease for the past ten years. His studies in 1998 note that GHB, as a pharmaceutical aid, can be much less toxic and much more effective than the leading pharmaceutical compound (]).{{Fact|date=February 2007}}</ref> In the United States, the ] permits the use of GHB under the trade name Xyrem to reduce the number of ] attacks in patients with ].<ref>In clinical trials Xyrem significantly reduced cataplexy attacks at a dose of 6000–9000mg per night. This is around three times the dose used recreationally, but almost all narcolepsy patients in the clinical trials were already stabilized on CNS stimulants such as ]; in patients not prescribed modafinil, this dosage could be dangerous and should be reduced appropriately. Also the prescribing information for Xyrem states that patients should take the dose immediately before going to bed, and then a second dose 3–4 hours later. The maximum dose taken at one time should not exceed 4500 mg. Patients with hepatic insufficiency (compromised liver function) have slower clearance of GHB and require reduced doses, typically half the normal dose. Xyrem oral solution is standardised to 500 mg Na.GHB / 1 mL water, buffered to pH 7.5 with malic acid. </ref> | |||
| {{clear right}} | |||
| When GHB is used in its sodium or potassium salt form, a significant quantity of excess sodium or potassium may be consumed, which should be taken into consideration by people with heart conditions, hypertension or compromised renal function. The ] of sodium GHB is considerably reduced when it is consumed with food, and so it is advised to wait at least two hours after eating before consuming the dose. Because of its strong sedative effects, patients should not drive or operate heavy machinery for at least six hours after taking sodium GHB. | |||
| ==Usage as a date-rape drug== | |||
| Adverse effects from Xyrem in clinical trials included: headache, nausea, dizziness, ], ], vomiting, ], confusion, ], ], ], ], ], and blurred vision. Out of the 717 patients and 182 healthy volunteers who took part in the trials (899 total), two of them died from drug overdoses, although only one of these involved GHB.<ref></ref> | |||
| ] | |||
| GHB became known to the general public as a ] by the late 1990s.<ref name="dea-daterape">{{cite web | url = http://www.usdoj.gov/dea/ongoing/daterapep.html | title = GHB, GBL and 1,4BD as Date Rape Drugs | access-date = 10 May 2012 | author = US Drug Enforcement Administration | archive-url = https://web.archive.org/web/20120510151441/http://www.justice.gov/dea//ongoing/daterapep.html | archive-date = 10 May 2012}}</ref><ref>{{cite news|agency=The Associated Press|title=Warning on Risk of 'Party Drug' Chemicals|url=https://www.nytimes.com/1999/05/12/us/warning-on-risk-of-party-drug-chemicals.html|work=The New York Times|date=12 May 1999|access-date=16 April 2018|archive-date=6 April 2023|archive-url=https://web.archive.org/web/20230406044951/https://www.nytimes.com/1999/05/12/us/warning-on-risk-of-party-drug-chemicals.html|url-status=live}}</ref><ref name="auto"/> GHB is colourless and odorless and has been described as "very easy to add to drinks".<ref name="jones"/> When consumed, the victim will quickly feel groggy and sleepy and may become unconscious. Upon recovery they may have an impaired ability to recall events that have occurred during the period of intoxication. In these situations evidence and the identification of the perpetrator of the rape is often difficult.<ref name="Németh2010">{{cite journal | vauthors = Németh Z, Kun B, Demetrovics Z | title = The involvement of gamma-hydroxybutyrate in reported sexual assaults: a systematic review | journal = Journal of Psychopharmacology | volume = 24 | issue = 9 | pages = 1281–7 | date = September 2010 | pmid = 20488831 | doi = 10.1177/0269881110363315 | s2cid = 25496192 }}</ref><ref>{{cite journal | vauthors = ElSohly MA, Salamone SJ | title = Prevalence of drugs used in cases of alleged sexual assault | journal = Journal of Analytical Toxicology | volume = 23 | issue = 3 | pages = 141–6 | year = 1999 | pmid = 10369321 | doi = 10.1093/jat/23.3.141 | doi-access = free }}</ref> | |||
| == Non-medical use== | |||
| ] | |||
| <!-- | |||
| Do not add street names below without references that they are in common use. | |||
| Otherwise they will be removed immediately. Misplaced Pages is not a dictionary, a jargon guide, or a collection of local trivia. | |||
| --> | |||
| GHB is a CNS ] used as an ]. It has many street names, including Liquid Ecstasy and Liquid X. At recreational doses, GHB can cause a state of ], increased enjoyment of movement and music, increased ], increased sociability and ]. At higher doses, GHB may induce ], ], ], ], visual disturbances, depressed ]ing, ], ], and death. The effects of GHB can last from 1.5 to 3 hours, or even longer if large doses have been consumed or if it is mixed with alcohol. | |||
| It is also difficult to establish how often GHB is used to facilitate rape as it is difficult to detect in a urine sample after a day, and many victims may only recall the rape some time after its occurrence; however, a 2006 study suggested that there was "no evidence to suggest widespread date rape drug use" in the UK, and that less than 2% of cases involved GHB, while 17% involved ],<ref>{{cite web | url=http://www.24dash.com/news/health/2006-11-16-No-evidence-to-suggest-widespread-date-rape-drug-use | archive-url=https://web.archive.org/web/20090108174731/http://www.24dash.com/news/Health/2006-11-16-No-evidence-to-suggest-widespread-date-rape-drug-use | url-status=dead | archive-date=8 January 2009 | title=No evidence to suggest widespread date rape drug use' | date=16 November 2006 | access-date=8 April 2014 }}</ref><ref>{{cite news | url=http://news.bbc.co.uk/1/hi/uk/6152646.stm | title=Date-rape drugs 'not widespread' | work=BBC News | date=16 November 2006 | access-date=8 April 2014 | archive-date=20 May 2007 | archive-url=https://web.archive.org/web/20070520122215/http://news.bbc.co.uk/1/hi/uk/6152646.stm | url-status=live }}</ref> and a survey in the Netherlands published in 2010 found that the proportion of drug-related rapes where GHB was used appeared to be greatly overestimated by the media.<ref name="Németh2010"/><ref>. udel.edu</ref><ref>{{cite web | url = https://www.independent.co.uk/news/world/europe/labs-making-daterape-drug-raided-863938.html | title = Labs making date-rape drug raided | archive-url = https://web.archive.org/web/20150925190920/http://www.independent.co.uk/news/world/europe/labs-making-daterape-drug-raided-863938.html | archive-date=25 September 2015 | work = The Independent | date = 10 July 2008 }}</ref> More recently, a study in Western Australia reviewed the pre-hospital context given in medical records around emergency department presentations with analytical confirmation of GHB exposure. This study found that most cases reported daily dosing and subsequent ] rather than their presentation being associated with date-rape.<ref>{{cite journal | vauthors = Smith JL, Greene S, McCutcheon D, Weber C, Kotkis E, Soderstrom J, Douglas B, Lenton S, Grigg J, Dessauer P, Ezard N, Fatovich DM | title = A multicentre case series of analytically confirmed gamma-hydroxybutyrate intoxications in Western Australian emergency departments: Pre-hospital circumstances, co-detections and clinical outcomes | journal = Drug and Alcohol Review | date = March 2024 | volume = 43 | issue = 4 | pages = 984–996 | pmid = 38426636 | doi = 10.1111/dar.13830 | doi-access = free }}</ref> | |||
| In general, the doses used recreationally are between 500 mg and 3000 mg, corresponding to approximately 0.5–3 mL of liquid if the concentration is 1 gram / 1 mL (which is not always the case). When used as a recreational drug, GHB may be found as the sodium or potassium salt, which is a white crystalline powder, or as GHB salt dissolved in water to form a clear solution - generally at a concentration of 1 gram / 1 mL and so is twice the strength of the Xyrem solution sold legally for medical use. The sodium salt of GHB has a thin, very salty, chemical taste.{{Fact|date=November 2007}} | |||
| There have been several high-profile cases of GHB as a date-rape drug that received national attention in the United States. In early 1999, a 15-year-old girl, ] of ], died from GHB poisoning. Reid's death inspired the legislation titled the "Hillory J. Farias and Samantha Reid Date-Rape Drug Prohibition Act of 2000". This is the law that made GHB a Schedule 1 controlled substance.<ref>{{cite web | vauthors = Martin JH | date = 16 January 2009 | url = http://www.thenewsherald.com/articles/2009/01/16/news/doc4970e3f098507043714937.txt | title = Remembering Samantha Reid: 10th anniversary of teen's GHB death | archive-url = https://web.archive.org/web/20160304093819/http://www.thenewsherald.com/articles/2009/01/16/news/doc4970e3f098507043714937.txt | archive-date=4 March 2016 | work = thenewsherald.com | access-date = 27 September 2012 }}</ref> In the United Kingdom, British serial killer ] administered GHB to his victims by adding it to drinks given to them, raping them, and murdering four of them in his flat in ], ].<ref name="BBC231116">{{cite news |url=https://www.bbc.co.uk/news/uk-england-38077859 |title=Stephen Port: Serial killer guilty of murdering four men |work=BBC News |date=23 November 2016}}</ref> | |||
| GHB salt dissolved in water is notoriously dangerous, as the concentration of GHB may not be known, and so the actual dose of GHB being consumed can be difficult to judge accurately. Since GHB sold for recreational use is subject to no standardisation it can be impossible to verify the actual concentration of GHB solution bought on the illicit market. Other salt forms such as calcium GHB and magnesium GHB have also been reported, but the sodium salt is by far the most common. | |||
| GHB can be detected in hair.<ref name="pmid12570228">{{cite journal | vauthors = Kintz P, Cirimele V, Jamey C, Ludes B | title = Testing for GHB in hair by GC/MS/MS after a single exposure. Application to document sexual assault | journal = Journal of Forensic Sciences | volume = 48 | issue = 1 | pages = 195–200 | date = January 2003 | pmid = 12570228 | doi = 10.1520/JFS2002209| url = http://www.hawaii.edu/hivandaids/Testing_for_GHB_in_Hair_by_GCMSMS_After_a_Single_Exposure_Doc_Sexual_Assault.pdf | archive-url = https://web.archive.org/web/20121224171516/http://www.hawaii.edu/hivandaids/Testing_for_GHB_in_Hair_by_GCMSMS_After_a_Single_Exposure_Doc_Sexual_Assault.pdf | archive-date=24 December 2012 }}</ref> Hair testing can be a useful tool in court cases or for the victim's own information.<ref>{{Cite web| title = Drink Speaks the Truth: Forensic Investigation of Drug Facilitated Sexual Assaults| url = http://www.forensicmag.com/articles/2013/06/drink-speaks-truth-forensic-investigation-drug-facilitated-sexual-assaults| date = 20 June 2013| access-date = 24 July 2014| archive-date = 14 March 2016| archive-url = https://web.archive.org/web/20160314222003/http://www.forensicmag.com/articles/2013/06/drink-speaks-truth-forensic-investigation-drug-facilitated-sexual-assaults| url-status = live}}</ref> Most over-the-counter urine test kits test only for date-rape drugs that are ]s, which GHB is not. To detect GHB in urine, the sample must be taken within four hours of GHB ingestion, and cannot be tested at home.<ref>{{cite journal | url=https://www.gtfch.org/cms/images/stories/media/tb/tb2015/Lott_et_al_2015.pdf |archive-url=https://web.archive.org/web/20160304082734/https://www.gtfch.org/cms/images/stories/media/tb/tb2015/Lott_et_al_2015.pdf |archive-date=4 March 2016 |url-status=live | title=Measurement of exogenous gamma-hydroxybutyric acid (GHB) in urine using isotope ratio mass spectrometry (IRMS) | vauthors = Lott S, Piper T, Mehling LM, Spottke A, Maas A, Thevis M, Madea B, Hess C | journal=Toxichem Krimtech | year=2015 | volume=82 | pages=264}}</ref> | |||
| Some chemicals convert to GHB in the stomach and blood. GBL, or ], is one such ]. Other prodrugs include ]. There may be additional toxicity concerns with these precursors. 1,4-B and GBL are normally found as pure liquids, although they may be mixed with other more harmful solvents when intended for industrial use, e.g., as ] or varnish thinner. | |||
| ==Adverse effects== | |||
| GHB can be produced in clandestine labs, and it is claimed that most of the GHB used in the US is illegally manufactured within its borders. While available as a prescription for sleep disorders in some other countries, GHB was banned (in the U.S.) by the FDA in 1990 because of the dangers associated with its use. However, on ], ] GHB was approved for treatment of cataplexy, often associated with narcolepsy. GHB is "colourless and odorless".<ref name="jones">Jones, C. Suspicious death related to gamma-hydroxybutyrate (GHB) toxicity (2001), Journal of Clinical Forensic Medicine Volume 8, Issue 2, June 2001, Pages 74-76.</ref> | |||
| ] study ranking various drugs (legal and illegal) based on statements by drug-harm experts. GHB was found to be the ninth overall most dangerous drug.<ref name="Nutt_2010">{{cite journal | vauthors = Nutt DJ, King LA, Phillips LD | title = Drug harms in the UK: a multicriteria decision analysis | journal = Lancet | volume = 376 | issue = 9752 | pages = 1558–1565 | date = November 2010 | pmid = 21036393 | doi = 10.1016/S0140-6736(10)61462-6 | s2cid = 5667719 | citeseerx = 10.1.1.690.1283 }}</ref>]] | |||
| ===Combination with alcohol=== | |||
| ===As a club scene or "rave" drug=== | |||
| In humans, GHB has been shown to reduce the elimination rate (thus increasing the elimination {{em|time}}) of alcohol. This may explain the respiratory arrest that has been reported after ingestion of both drugs.<ref>{{cite journal | vauthors = Poldrugo F, Addolorato G | title = The role of gamma-hydroxybutyric acid in the treatment of alcoholism: from animal to clinical studies | journal = Alcohol and Alcoholism | volume = 34 | issue = 1 | pages = 15–24 | year = 1999 | pmid = 10075397 | doi = 10.1093/alcalc/34.1.15 | doi-access = free }}</ref> A review of the details of 194 deaths attributed to or related to GHB over a ten-year period found that most were from respiratory depression caused by interaction with alcohol or other drugs.<ref>Zvosec et al. . Web.archive.org (3 December 2007). Retrieved on 24 December 2011.</ref> | |||
| {{Refimprovesect|date=July 2007}} | |||
| ===Deaths=== | |||
| Since the 1970s ], ] have used a range of drugs to enhance their experience on the dance floor such as amyl nitrite "]" and ]; in the 1990s, newer "club drugs" became popular, such as ] and Ecstasy (]). Like these other "club drugs," GHB is taken because users feel that it enhances the experience of being in a club or at a party; GHB is sometimes referred to as ''liquid ecstasy'' due to its tendency to produce euphoria and sociability and its use in the dance party scene. | |||
| One publication has investigated 226 deaths attributed to GHB.<ref>{{cite journal | vauthors = Zvosec DL, Smith SW, Porrata T, Strobl AQ, Dyer JE | title = Case series of 226 γ-hydroxybutyrate-associated deaths: lethal toxicity and trauma | journal = The American Journal of Emergency Medicine | volume = 29 | issue = 3 | pages = 319–32 | date = March 2011 | pmid = 20825811 | doi = 10.1016/j.ajem.2009.11.008 }}</ref> Of the 226 deaths included, 213 had a cardiorespiratory arrest and 13 had fatal accidents. Seventy-one of these deaths (34%) had no co-intoxicants. Postmortem blood GHB was 18–4400 mg/L (median=347) in deaths negative for co-intoxicants. | |||
| One report has suggested that sodium oxybate overdose might be fatal, based on deaths of three patients who had been prescribed the drug.<ref>{{cite journal | vauthors = Zvosec DL, Smith SW, Hall BJ | title = Three deaths associated with use of Xyrem | journal = Sleep Medicine | volume = 10 | issue = 4 | pages = 490–93 | date = April 2009 | pmid = 19269893 | doi = 10.1016/j.sleep.2009.01.005 }}</ref> However, for two of the three cases, post-mortem GHB concentrations were 141 and 110 mg/L, which is within the expected range of concentrations for GHB after death, and the third case was a patient with a history of intentional drug overdose.<ref>{{cite journal | vauthors = Feldman NT | title = Xyrem safety: the debate continues | journal = Sleep Medicine | volume = 10 | issue = 4 | pages = 405–06 | date = April 2009 | pmid = 19332385 | doi = 10.1016/j.sleep.2009.02.002 }}</ref> The toxicity of GHB has been an issue in criminal trials, as in the death of ], where the defense argued that death was due to GHB, not murder. | |||
| ===As a date rape drug=== | |||
| The drug has been referred to in the media as a ], in much the same way as ] and ]. As it is colourless and odorless,<ref name="jones"/> it has been described as "very easy to add to drinks".<ref name="jones"/> GHB has been used in many cases of drug-related sexual assault, usually when the victim is vulnerable due to intoxication with a sedative, generally alcohol or more rarely cannabis, and as such are less likely to notice a strange taste to his or her drink.<ref> http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=10369321&ordinalpos=27&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref> However it is difficult to establish how often GHB is used to facilitate rape as it is difficult to detect in a urine sample after a day, and many victims may not recall the rape until some time after this.<ref>http://www.udel.edu/wellspring/SOS/drugs.htm</ref> GHB produced as a sodium salt (sodium oxybate) may provide a noticeable salty character to the drink, although individual sensitivity to the taste of salt varies<ref>http://www.ajcn.org/cgi/content/abstract/35/3/510</ref>. GHB can also be produced as different salts, some of which may not have a taste as distinctive as the sodium salt (e.g., magnesium oxybate), or much less commonly in the unstable free-acid form.<ref>http://www.blackwell-synergy.com/doi/abs/10.1111/j.1556-4029.2006.00074.x?journalCode=jfo</ref> | |||
| GHB is produced in the body in very small amounts, and blood levels may climb after death to levels in the range of 30–50 mg/L.<ref>{{cite journal | vauthors = Zvosec DL, Smith SW | title = Response to Editorial: "Xyrem safety: The debate continues" | journal = Sleep Medicine | volume = 11 | issue = 1 | pages = 108; author reply 108–09 | date = January 2010 | pmid = 19959395 | doi = 10.1016/j.sleep.2009.08.004 }}</ref> Levels higher than this are found in GHB deaths. Levels lower than this may be due to GHB or to postmortem endogenous elevations. | |||
| ===Use by bodybuilders=== | |||
| Some athletes and bodybuilders also use GHB, as GHB has been shown to elevate human growth hormone in vivo.<ref> http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=9373886&ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVAbstractPlus</ref> | |||
| The growth hormone elevating effects of GHB are mediated through muscarinic acetylcholine receptors and can be prevented by prior administration of pirenzepine, a muscarinic acetylcholine receptor blocking agent.<ref>{{cite web|title=Muscarinic cholinergic mediation of the GH response to gamma-hydroxybutyric acid: neuroendocrine evidence in normal and parkinsonian subjects|url=http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TBX-3XWJKG7-6&_user=10&_coverDate=02%2F29%2F2000&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=e5444781501a8528283aaba4bf91bc30}} </ref> | |||
| ===Neurotoxicity=== | |||
| As certain ] salts have been shown to elevate growth hormone ]<ref>http://www.nature.com/npp/journal/v11/n4/abs/1380135a.html</ref>, being GHB is metabolized into succinate some people have suggested this may play a role in the growth hormone elevations from GHB. | |||
| In multiple studies, GHB has been found to impair ], ], ] and ] in rats with chronic ad{{shy}}min{{shy}}is{{shy}}tra{{shy}}tion.<ref name="pmid15582677">{{cite journal | vauthors = Sircar R, Basak A | title = Adolescent gamma-hydroxybutyric acid exposure decreases cortical N-methyl-D-aspartate receptor and impairs spatial learning | journal = Pharmacology, Biochemistry, and Behavior | volume = 79 | issue = 4 | pages = 701–08 | date = December 2004 | pmid = 15582677 | doi = 10.1016/j.pbb.2004.09.022 | s2cid = 31736568 }}</ref><ref name="pmid17296081">{{cite journal | vauthors = García FB, Pedraza C, Arias JL, Navarro JF | title = | language = es | journal = Psicothema | volume = 18 | issue = 3 | pages = 519–24 | date = August 2006 | pmid = 17296081 }}</ref><ref name="pmid18991885">{{cite journal | vauthors = Sircar R, Basak A, Sircar D | title = Gamma-hydroxybutyric acid-induced cognitive deficits in the female adolescent rat | journal = Annals of the New York Academy of Sciences | volume = 1139 | issue = 1 | pages = 386–89 | date = October 2008 | pmid = 18991885 | doi = 10.1196/annals.1432.044 | bibcode = 2008NYASA1139..386S | s2cid = 1823886 }}</ref> These effects are associated with decreased ] expression in the ] and possibly other areas as well.<ref name="pmid15582677"/> In addition, the neu{{shy}}ro{{shy}}tox{{shy}}icity appears to be caused by ].<ref name="pmid17197055">{{cite journal | vauthors = Sgaravatti AM, Sgarbi MB, Testa CG, Durigon K, Pederzolli CD, Prestes CC, Wyse AT, Wannmacher CM, Wajner M, Dutra-Filho CS | title = Gamma-hydroxybutyric acid induces oxidative stress in cerebral cortex of young rats | journal = Neurochemistry International | volume = 50 | issue = 3 | pages = 564–70 | date = February 2007 | pmid = 17197055 | doi = 10.1016/j.neuint.2006.11.007 | s2cid = 43049617 }}</ref><ref name="pmid19296210">{{cite journal | vauthors = Sgaravatti AM, Magnusson AS, Oliveira AS, Mescka CP, Zanin F, Sgarbi MB, Pederzolli CD, Wyse AT, Wannmacher CM, Wajner M, Dutra-Filho CS | title = Effects of 1,4-butanediol administration on oxidative stress in rat brain: study of the neurotoxicity of gamma-hydroxybutyric acid in vivo | journal = Metabolic Brain Disease | volume = 24 | issue = 2 | pages = 271–82 | date = June 2009 | pmid = 19296210 | doi = 10.1007/s11011-009-9136-7 | s2cid = 13460935 }}</ref> | |||
| There is however currently no evidence to show that succinate plays any role in the growth hormone elevations from GHB. | |||
| ===Addiction=== | |||
| === Endogenous production by the body === | |||
| Addiction occurs when repeated drug use disrupts the normal balance of brain circuits that control rewards, memory and cognition, ultimately leading to compulsive drug taking.<ref>Department of Health and Human Services, SAMHSA Office of Applied Studies 2005 National Survey on Drug Use and Health (ages 12 years and up); American Heart Association; Johns Hopkins University study, Principles of Addiction Medicine; Psychology Today; National Gambling Impact Commission Study; National Council on Problem Gambling; Illinois Institute for Addiction Recovery; Society for Advancement of Sexual Health; All Psych Journal</ref><ref>. Time</ref> | |||
| Cells produce GHB by reduction of ]. This enzyme appears to be induced by cAMP levels<ref>http://lib.bioinfo.pl/pmid:9692734</ref>, meaning substances that elevate cAMP, such as ] and ], may increase GHB synthesis and release. People with the disorder known as ], also known as ], have elevated levels of GHB in their ], blood plasma and ].<ref></ref> | |||
| Rats forced to consume massive doses of GHB will intermittently prefer GHB solution to water.<ref>{{cite journal | vauthors = Colombo G, Agabio R, Balaklievskaia N, Diaz G, Lobina C, Reali R, Gessa GL | title = Oral self-administration of gamma-hydroxybutyric acid in the rat | journal = European Journal of Pharmacology | volume = 285 | issue = 1 | pages = 103–07 | date = October 1995 | pmid = 8846805 | doi = 10.1016/0014-2999(95)00493-5 }}</ref><ref>{{cite web | url = http://www.lycaeum.org/~ghbfaq/dangerous.html | title = Is GHB toxic? Addictive? Dangerous? | archive-url = https://web.archive.org/web/20110127050233/http://www.lycaeum.org/~ghbfaq/dangerous.html | archive-date=27 January 2011 | work = lycaeum.org }}</ref> | |||
| The precise function of GHB in the body is not clear. It is known however that the brain expresses a large amount of receptors that are activated by GHB.<ref>http://www.fasebj.org/cgi/content/full/17/12/1691</ref> These receptors are excitatory and not responsible for the sedative effects of GHB - they have been shown to elevate the principle excitatory neurotransmitter - ].<ref>http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=14535954&ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref> | |||
| The benzamide antipsychotics - ], ] - have been shown to bind to this receptor in vivo<ref>http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7914168&dopt=Abstract</ref>. Other antipsychotics were tested and were not found to have an affinity for this receptor. | |||
| ===Withdrawal=== | |||
| It is a precursor to ], ] and ] in certain brain areas.<ref>http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=10381791&ordinalpos=13&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref> | |||
| GHB has also been associated with a ] of ], anxiety, and ] that usually resolves within three to twenty-one days.<ref name="schep"/><ref name="autogenerated1997">{{cite journal | vauthors = Galloway GP, Frederick SL, Staggers FE, Gonzales M, Stalcup SA, Smith DE | title = Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence | journal = Addiction | volume = 92 | issue = 1 | pages = 89–96 | date = January 1997 | pmid = 9060200 | doi = 10.1111/j.1360-0443.1997.tb03640.x }}</ref><ref name="J Pharm Pharmaceut Sci">{{cite web |url=https://www.ualberta.ca/~csps/JPPS4(2)/M.Okun/GHB.htm |title=GHB: An Important Pharmacologic and Clinical Update |publisher=Ualberta.ca |access-date=1 August 2010 |archive-date=4 March 2016 |archive-url=https://web.archive.org/web/20160304115225/http://www.ualberta.ca/~csps/JPPS4(2)/M.Okun/GHB.htm |url-status=live }}</ref> The withdrawal syndrome can be severe producing acute delirium and may require hospitalization in an intensive care unit for management.<ref name="schep"/> Management of GHB dependence involves considering the person's age, comorbidity and the pharmacological pathways of GHB.<ref name="pmid28186869">{{cite journal | vauthors = Santos C, Olmedo RE | title = Sedative-Hypnotic Drug Withdrawal Syndrome: Recognition And Treatment | journal = Emergency Medicine Practice | volume = 19 | issue = 3 | pages = 1–20 | date = March 2017 | pmid = 28186869 }}</ref> The mainstay of treatment for severe withdrawal is supportive care and ] for control of acute ], but larger doses are often required compared to acute delirium of other causes (e.g. > 100 mg/d of ]). ] has been suggested as an alternative or adjunct to benzodiazepines based on anecdotal evidence and some animal data.<ref>{{cite journal | vauthors = LeTourneau JL, Hagg DS, Smith SM | title = Baclofen and gamma-hydroxybutyrate withdrawal | journal = Neurocritical Care | volume = 8 | issue = 3 | pages = 430–33 | year = 2008 | pmid = 18266111 | pmc = 2630388 | doi = 10.1007/s12028-008-9062-2 }}</ref> However, there is less experience with the use of baclofen for GHB withdrawal, and additional research in humans is needed. Baclofen was first suggested as an adjunct because benzodiazepines do not affect GABA<sub>B</sub> receptors and therefore have no ] with GHB while baclofen, which works via GABA<sub>B</sub> receptors, is cross-tolerant with GHB and may be more effective in alleviating withdrawal effects of GHB.<ref name="Carter-2009">{{cite journal | vauthors = Carter LP, Koek W, France CP | title = Behavioral analyses of GHB: receptor mechanisms | journal = Pharmacology & Therapeutics | volume = 121 | issue = 1 | pages = 100–14 | date = January 2009 | pmid = 19010351 | pmc = 2631377 | doi = 10.1016/j.pharmthera.2008.10.003 }}</ref> | |||
| GHB withdrawal is not widely discussed in textbooks and some psychiatrists, general practitioners, and even hospital emergency physicians may not be familiar with this withdrawal syndrome.<ref name="van Noorden-">{{cite journal | vauthors = van Noorden MS, van Dongen LC, Zitman FG, Vergouwen TA | title = Gamma-hydroxybutyrate withdrawal syndrome: dangerous but not well-known | journal = General Hospital Psychiatry | volume = 31 | issue = 4 | pages = 394–96 | year = 2009 | pmid = 19555805 | doi = 10.1016/j.genhosppsych.2008.11.001 }}</ref> | |||
| GHB has neuroprotective properties and has been found to protects cells from ].<ref>http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=12965243&ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum</ref> | |||
| ==Overdose== | |||
| === As a natural fermentation by-product === | |||
| Overdose of GHB can sometimes be difficult to treat because of its multiple effects on the body.<ref name="emedicineonGHB"/><ref name="allenalsalim">{{cite journal | vauthors = Allen L, Alsalim W | title = Best evidence topic report. Gammahydroxybutyrate overdose and physostigmine | journal = Emergency Medicine Journal | volume = 23 | issue = 4 | pages = 300–01 | date = April 2006 | pmid = 16549578 | pmc = 2579509 | doi = 10.1136/emj.2006.035139 }}</ref><ref name="intubationtreatment">{{cite journal | vauthors = Michael H, Harrison M | title = Best evidence topic report: endotracheal intubation in gamma-hydroxybutyric acid intoxication and overdose | journal = Emergency Medicine Journal | volume = 22 | issue = 1 | pages = 43 | date = January 2005 | pmid = 15611542 | pmc = 1726538 | doi = 10.1136/emj.2004.021154 }}</ref> GHB tends to cause rapid unconsciousness at doses above 3500 mg, with single doses over 7000 mg often causing life-threatening ], and higher doses still inducing ] and ]. Other side-effects include ] (especially when combined with ]), and nausea/vomiting (especially when combined with alcohol).<ref name="schep"/> | |||
| GHB is also produced as a result of fermentation and so is found in small quantities in some beers and wines, particularly fruit wines. However, the amount of GHB found in wine is insignificant and not sufficient to produce any effects. <ref> Elliott S, Burgess V. The presence of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) in alcoholic and non-alcoholic beverages. Forensic Science International. 2005 July 16;151(2-3):289-92. </ref> | |||
| The greatest life threat due to GHB overdose (with or without other substances) is respiratory arrest.<ref name="schep"/><ref>{{cite journal | vauthors = Morse BL, Vijay N, Morris ME | title = γ-Hydroxybutyrate (GHB)-induced respiratory depression: combined receptor-transporter inhibition therapy for treatment in GHB overdose | journal = Molecular Pharmacology | volume = 82 | issue = 2 | pages = 226–235 | date = August 2012 | pmid = 22561075 | pmc = 3400846 | doi = 10.1124/mol.112.078154 }}</ref> Other relatively common causes of death due to GHB ingestion include ] of vomitus, positional asphyxia, and trauma sustained while intoxicated (e.g., motor vehicle accidents while driving under the influence of GHB).<ref>{{cite journal | vauthors = Zvosec DL, Smith SW, Porrata T, Strobl AQ, Dyer JE | title = Case series of 226 γ-hydroxybutyrate-associated deaths: lethal toxicity and trauma | journal = The American Journal of Emergency Medicine | volume = 29 | issue = 3 | pages = 319–332 | date = March 2011 | pmid = 20825811 | doi = 10.1016/j.ajem.2009.11.008 }}</ref> The risk of aspiration pneumonia and positional asphyxia risk can be reduced by laying the patient down in the ]. People are most likely to vomit as they become unconscious, and as they wake up. It is important to keep the victim awake and moving; the victim must not be left alone due to the risk of death through vomiting. Frequently the victim will be in a good mood but this does not mean the victim is not in danger. GHB overdose is a medical emergency and immediate assessment in an emergency department is needed. | |||
| == Dangers == | |||
| As with pure alcohol, the ] of GHB is very steep, and "proper" dosing of illegal GHB can be difficult since it often comes as a salt dissolved in water, and the actual amount of GHB and/or other additives per "capful" can vary. Legal GHB comes in standardized doses and is free from contaminants, so it is much safer (cf. legal alcohol vs. ]). Also, like pure alcohol, small doses of GHB are considered safe, but high doses can cause ], ], ], suppression of the ], and ]. These effects vary between persons and are dose-dependent. Synergy of its sedative effects are seen when combined with other ] such as alcohol, ]s (e.g., ]), ]s, and others. | |||
| Convulsions from GHB can be treated with the ]s ] or ].<ref name="schep"/> Even though these benzodiazepines are also CNS depressants, they primarily modulate GABA<sub>A</sub> receptors whereas GHB is primarily a GABA<sub>B</sub> receptor agonist, and so do not worsen CNS depression as much as might be expected.<ref>{{cite book |vauthors=Allen MJ, Sabir S, Sharma S |chapter=GABA Receptor |date=2022 |chapter-url=http://www.ncbi.nlm.nih.gov/books/NBK526124/ |title=StatPearls |place=Treasure Island (FL) |publisher=StatPearls Publishing |pmid=30252380 |access-date=21 October 2022 |archive-date=1 December 2022 |archive-url=https://web.archive.org/web/20221201103049/https://www.ncbi.nlm.nih.gov/books/NBK526124/ |url-status=live }}</ref> | |||
| Another complication is the difference in ] between GHB and its two prodrugs, 1,4-B and GBL. ] is converted into GHB in the body by two enzymes ] and ], which gives it a delayed onset of effects and a longer duration of action. GHB is then further metabolised, again by alcohol dehydrogenase and aldehyde dehydrogenase, into the inactive ]. | |||
| Because of the faster and more complete absorption of GBL relative to GHB, its dose-response curve is steeper, and overdoses of GBL tend to be more dangerous and problematic than overdoses involving only GHB or 1,4-B. Any GHB/GBL overdose is a ] and should be cared for by appropriately trained personnel. | |||
| If alcohol has also been consumed this can saturate the dehydrogenase enzymes and so delays the conversion of 1,4-B into GHB, meaning that 1,4-B takes much longer to take effect and people may re-dose thinking it hasn't done anything, leading to an accidental overdose later on once it finally takes effect. 1,4-B itself can also contribute to the enzyme saturation, so, when alcohol and 1,4-B are consumed together, it produces a complex and somewhat unpredictable interaction between the varying levels of alcohol, 1,4-B and GHB present in the body. Alcohol also makes the GHB last longer in the body by competing for dehydrogenase enzymes, and hence delaying the conversion of GHB into succinate. | |||
| A newer synthetic drug, ], which acts as a selective GABA<sub>B</sub> antagonist, quickly reverses GHB overdose in mice.<ref name="mousetreatment">{{cite journal | vauthors = Carai MA, Colombo G, Gessa GL | title = Resuscitative effect of a gamma-aminobutyric acid B receptor antagonist on gamma-hydroxybutyric acid mortality in mice | journal = Annals of Emergency Medicine | volume = 45 | issue = 6 | pages = 614–19 | date = June 2005 | pmid = 15940094 | doi = 10.1016/j.annemergmed.2004.12.013 }}</ref> However, this treatment has yet to be tried in humans, and it is unlikely that it will be researched for this purpose in humans due to the illegal nature of clinical trials of GHB and the lack of medical indemnity coverage inherent in using an untested treatment for a life-threatening overdose.<ref name="auto"/> | |||
| The other precursor ] (GBL) is rapidly converted into GHB by ] enzymes found in the blood. GBL is more lipophilic (fat soluble) than GHB, and so is absorbed faster and has higher bioavailability; the paradox is that this can mean that GBL has a faster onset of effects than GHB itself, even though it is a ]. The levels of lactamase enzyme can vary between individuals, and GBL is not active in its own right, so people who have never tried GBL before may have delayed or fewer effects than expected; however, once someone has taken GBL a few times, the production of lactamase enzymes is increased and he/she will feel the effects like normal. | |||
| ===Detection of use=== | |||
| Because of these pharmacokinetic differences, 1,4-B tends to be slightly less potent, slower to take effect but longer-acting than GHB, whereas GBL tends to be more potent and faster-acting than GHB, and has around the same duration. | |||
| GHB may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized patients,<ref name="schep"/> to provide evidence in an impaired driving, or to assist in a medicolegal death investigation. Blood or plasma GHB concentrations are usually in a range of 50–250 mg/L in persons receiving the drug therapeutically (during general anesthesia), 30–100 mg/L in those arrested for impaired driving, 50–500 mg/L in acutely intoxicated patients and 100–1000 mg/L in victims of fatal overdosage. Urine is often the preferred specimen for routine drug abuse monitoring purposes. Both ] (GBL) and ] are converted to GHB in the body.<ref>{{cite journal | vauthors = Couper FJ, Thatcher JE, Logan BK | title = Suspected GHB overdoses in the emergency department | journal = Journal of Analytical Toxicology | volume = 28 | issue = 6 | pages = 481–84 | date = September 2004 | pmid = 15516299 | doi = 10.1093/jat/28.6.481 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Marinetti LJ, Isenschmid DS, Hepler BR, Kanluen S | title = Analysis of GHB and 4-methyl-GHB in postmortem matrices after long-term storage | journal = Journal of Analytical Toxicology | volume = 29 | issue = 1 | pages = 41–47 | year = 2005 | pmid = 15808012 | doi = 10.1093/jat/29.1.41 | doi-access = free }}</ref><ref>R. Baselt, ''Disposition of Toxic Drugs and Chemicals in Man'', 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 680–84.</ref> | |||
| In January 2016, it was announced scientists had developed a way to detect GHB, among other things, in saliva.<ref>{{cite news|url=http://www.bbc.co.uk/newsbeat/article/35262515/new-spit-test-for-date-rape-drug-developed-in-the-uk|title=New spit test for 'date rape' drug developed in the UK|work=BBC News|date=August 2016|access-date=9 January 2016|archive-date=8 November 2020|archive-url=https://web.archive.org/web/20201108135545/http://www.bbc.co.uk/newsbeat/article/35262515/new-spit-test-for-date-rape-drug-developed-in-the-uk|url-status=live}}</ref> | |||
| Alcohol worsens both CNS depression and vomiting, so combining alcohol with GHB or its precursors can be particularly dangerous. Another factor to be considered is that people who drink alcohol regularly tend to induce expression of their dehydrogenase enzymes, and thus have higher levels of these enzymes than people that do not drink alcohol regularly; this means that regular alcohol drinkers will both convert 1,4-B into GHB more rapidly and also break down GHB into succinate faster than people that do not drink alcohol. This multitude of different factors can make the interactions between 1,4-B, GHB and alcohol very complicated and highly variable between different individuals. | |||
| ==Endogenous production== | |||
| Death while using GHB is most likely when it is combined with alcohol or other depressant drugs; however, as with all substances, an overdose of GHB alone may be lethal. A review of the details of 194 deaths attributed to or related to GHB over a ten-year period<ref>Zvosec et al. American Academy of Forensic Science in Seattle, 2006 </ref> found that most were from respiratory depression caused by interaction with alcohol or other drugs; several were from choking on vomit and asphyxiating; remaining causes of death included motor vehicle and other accidents. The review included 70 cases where high levels of GHB were found post-mortem without concomitant ingestion of other drugs or alcohol. | |||
| Cells produce GHB by reduction of ] via ] (SSR). This enzyme appears to be induced by cAMP levels,<ref>{{cite journal | vauthors = Kemmel V, Taleb O, Perard A, Andriamampandry C, Siffert JC, Mark J, Maitre M | title = Neurochemical and electrophysiological evidence for the existence of a functional gamma-hydroxybutyrate system in NCB-20 neurons | journal = Neuroscience | volume = 86 | issue = 3 | pages = 989–1000 | date = October 1998 | pmid = 9692734 | doi = 10.1016/S0306-4522(98)00085-2 | s2cid = 21001043 }}</ref> meaning substances that elevate cAMP, such as ] and ], may increase GHB synthesis and release. Conversely, endogeneous GHB production in those taking ] will be inhibited via inhibition of the conversion from succinic acid semialdehyde to GHB.<ref>{{cite book | vauthors = Löscher W | author-link1 = Wolfgang Löscher | chapter = Valproic Acid: Mechanism of Action | chapter-url = https://books.google.com/books?id=HAOY0qG-vAYC&q=vpa+inhibition+of+ssa+to+GHB&pg=PA774 | veditors = Levy RH, Mattson RH, Meldrum BS, Perucca E | title = Antiepileptic drugs | date = 2002 | publisher = Lippincott Williams & Wilkins | location = Philadelphia | isbn = 978-0-7817-2321-3 | edition = 5th | page = 774 | access-date = 16 August 2021 | archive-date = 18 August 2023 | archive-url = https://web.archive.org/web/20230818072407/https://books.google.com/books?id=HAOY0qG-vAYC&q=vpa+inhibition+of+ssa+to+GHB&pg=PA774 | url-status = live }}</ref> People with the disorder known as succinic semialdehyde dehydrogenase deficiency, also known as ], have elevated levels of GHB in their ], blood plasma and ].<ref>National Organization for Rare Disorders. {{Webarchive|url=https://web.archive.org/web/20230406000025/http://rarediseases.org/search/rdbdetail_abstract.html?disname=Succinic%20Semialdehyde%20Dehydrogenase%20Deficiency |date=6 April 2023 }}. Retrieved 6 March 2010.</ref> | |||
| The precise function of GHB in the body is not clear. It is known, however, that the brain expresses a large number of receptors that are activated by GHB.<ref>{{cite journal | vauthors = Andriamampandry C, Taleb O, Viry S, Muller C, Humbert JP, Gobaille S, Aunis D, Maitre M | s2cid = 489179 | title = Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator gamma-hydroxybutyrate (GHB) | journal = FASEB Journal | volume = 17 | issue = 12 | pages = 1691–93 | date = September 2003 | pmid = 12958178 | doi = 10.1096/fj.02-0846fje | doi-access = free }}</ref> These receptors are excitatory, however, and therefore not responsible for the sedative effects of GHB; they have been shown to elevate the principal excitatory neurotransmitter, ].<ref name = "nsngtu"/> The ] antipsychotics—], ], etc.—have been shown to bind to these GHB-activated receptors in vivo.<ref>{{cite journal | vauthors = Maitre M, Ratomponirina C, Gobaille S, Hodé Y, Hechler V | title = Displacement of gamma-hydroxybutyrate binding by benzamide neuroleptics and prochlorperazine but not by other antipsychotics | journal = European Journal of Pharmacology | volume = 256 | issue = 2 | pages = 211–14 | date = April 1994 | pmid = 7914168 | doi = 10.1016/0014-2999(94)90248-8 }}</ref> Other antipsychotics were tested and were not found to have an affinity for this receptor. | |||
| Determining conclusively whether someone's death was caused by GHB is very difficult because a lab test will always detect the presence of some GHB in the human body, and levels of GHB can vary in the same individual depending on what part of the body is tested. GHB is a naturally-occurring substance that is always present in everyone, but little research has been done on what levels are normal in what parts of the body at what times. | |||
| GHB is a precursor to ], glutamate, and ] in certain brain areas.<ref>{{cite journal | vauthors = Gobaille S, Hechler V, Andriamampandry C, Kemmel V, Maitre M | title = gamma-Hydroxybutyrate modulates synthesis and extracellular concentration of gamma-aminobutyric acid in discrete rat brain regions in vivo | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 290 | issue = 1 | pages = 303–09 | date = July 1999 | pmid = 10381791 }}</ref> | |||
| There have been no systematic studies into the effects of GHB if taken chronically in humans, and hence whether prolonged use of GHB causes any bodily harm remains unknown. A ] parliamentary committee commissioned report found the use of GHB to be less dangerous than tobacco and alcohol in social harms, physical harm and addiction.<ref>Science and Technology Committee Report (page 176), 2006). </ref> | |||
| In spite of its demonstrated neurotoxicity, (see ], above), GHB has neuroprotective properties, and has been found to protect cells from ].<ref>{{cite journal | vauthors = Ottani A, Saltini S, Bartiromo M, Zaffe D, Renzo Botticelli A, Ferrari A, Bertolini A, Genedani S | title = Effect of gamma-hydroxybutyrate in two rat models of focal cerebral damage | journal = Brain Research | volume = 986 | issue = 1–2 | pages = 181–90 | date = October 2003 | pmid = 12965243 | doi = 10.1016/S0006-8993(03)03252-9 | s2cid = 54374774 }}</ref> | |||
| === Treatment of overdose <ref name="allenalsalim">{{cite journal | author = Allen, L. | coauthors = Alsalim, W. | date = ] | title = Gammahydroxybutyrate overdose and physostigmine | journal = ] | volume = 23 | issue = 4 | pages = 300 | doi = 10.1136/emj.2006.035139 }}</ref><ref name="intubationtreatment">{{cite journal | author = Michael, H. | coauthors = Harrison, M. | date = ] | title = Endotracheal intubation in γ-hydroxybutyric acid intoxication and overdose | journal = ] | volume = 22 | issue = 1 | pages = 43 | doi = 10.1136/emj.2004.021154 }}</ref><ref name="emedicineonGHB"/>=== | |||
| Overdose of GHB can be difficult to treat because of its multiple effects on the body. GHB tends to cause rapid unconsciousness at doses above 3500 mg, with single doses over 7000 mg often causing life-threatening ], and higher doses still can induce ] and consequent heart failure. Because of the faster and more complete absorption of GBL relative to GHB, its dose-response curve is steeper, and overdoses of GBL tend to be more dangerous and problematic than overdoses involving only GHB or 1,4-B. | |||
| ==Natural fermentation by-product== | |||
| As well as causing these depressant effects, GHB overdose also often produces twitches or convulsions, especially when combined with stimulants such as ]. Also GHB tends to cause nausea and vomiting, particularly when combined with alcohol; so a patient may be simultaneously unconscious, vomiting, and convulsing. | |||
| GHB is also produced as a result of fermentation and so is found in small quantities in some beers and wines, in particular fruit wines. The amount found in wine is pharmacologically insignificant and not sufficient to produce psychoactive effects.<ref>{{cite journal | vauthors = Elliott S, Burgess V | title = The presence of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) in alcoholic and non-alcoholic beverages | journal = Forensic Science International | volume = 151 | issue = 2–3 | pages = 289–92 | date = July 2005 | pmid = 15939164 | doi = 10.1016/j.forsciint.2005.02.014 }}</ref> | |||
| ==Pharmacology== | |||
| Overdoses wherein the patient has consumed GHB along with both alcohol and amphetamine-based stimulants are often particularly problematic; the stimulants may cause the patient to slip in and out of consciousness and he/she may be confused and combative, fighting off medical staff while half-awake before lapsing back into unconsciousness again. Care should be taken when attempting to sweep foreign bodies out of the mouth of a patient that presents with a suspected GHB overdose. The patient may bite very hard, and sometimes will not let go.{{Fact|date=November 2007}} | |||
| GHB has at least two distinct binding sites in the central nervous system.<ref>{{cite journal | vauthors = Wu Y, Ali S, Ahmadian G, Liu CC, Wang YT, Gibson KM, Calver AR, Francis J, Pangalos MN, Carter Snead O | title = Gamma-hydroxybutyric acid (GHB) and gamma-aminobutyric acidB receptor (GABABR) binding sites are distinctive from one another: molecular evidence | journal = Neuropharmacology | volume = 47 | issue = 8 | pages = 1146–56 | date = December 2004 | pmid = 15567424 | doi = 10.1016/j.neuropharm.2004.08.019 | s2cid = 54233206 }}</ref> GHB acts as an ] at the ] ]<ref>{{cite journal | vauthors = Cash CD, Gobaille S, Kemmel V, Andriamampandry C, Maitre M | title = Gamma-hydroxybutyrate receptor function studied by the modulation of nitric oxide synthase activity in rat frontal cortex punches | journal = Biochemical Pharmacology | volume = 58 | issue = 11 | pages = 1815–19 | date = December 1999 | pmid = 10571257 | doi = 10.1016/S0006-2952(99)00265-8 }}</ref><ref name="mechanism">{{cite journal | vauthors = Maitre M, Humbert JP, Kemmel V, Aunis D, Andriamampandry C | title = | language = fr | journal = Médecine/Sciences | volume = 21 | issue = 3 | pages = 284–89 | date = March 2005 | pmid = 15745703 | doi = 10.1051/medsci/2005213284 | doi-access = free }}</ref> and as a weak agonist at the ] ] receptor.<ref name="mechanism" /> GHB is a naturally occurring substance that acts in a similar fashion to some ] in the mammalian brain.<ref>{{cite journal | vauthors = Waszkielewicz A, Bojarski J | title = Gamma-hydrobutyric acid (GHB) and its chemical modifications: a review of the GHBergic system | journal = Polish Journal of Pharmacology | volume = 56 | issue = 1 | pages = 43–49 | year = 2004 | pmid = 15047976 | url = http://www.if-pan.krakow.pl/pjp/pdf/2004/1_43.pdf | access-date = 3 June 2008 | archive-date = 6 August 2021 | archive-url = https://web.archive.org/web/20210806054129/http://if-pan.krakow.pl/pjp/pdf/2004/1_43.pdf | url-status = live }}</ref> GHB is probably synthesized from GABA in GABAergic ], and released when the neurons fire.<ref name="mechanism" /> | |||
| GHB has been found to activate ]ergic ]s in the ].<ref>{{cite journal | vauthors = McGregor IS, Callaghan PD, Hunt GE | title = From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? | journal = British Journal of Pharmacology | volume = 154 | issue = 2 | pages = 358–68 | date = May 2008 | pmid = 18475254 | pmc = 2442436 | doi = 10.1038/bjp.2008.132 }}</ref> | |||
| The most likely risk of death from GHB overdose is inhalation of vomit while unconscious. This risk can be partially prevented by laying the patient down in the ]. People are most likely to vomit as they become unconscious, and as they wake up. This is best managed in a hospital setting, but, if the patient is not in a hospital, it is very important that someone stays with the patient until he/she becomes fully unconscious, to keep the patient in the recovery position and to check how deeply unconscious the he/she becomes. | |||
| If taken orally, GABA itself does not effectively cross the ].<ref>{{cite journal | vauthors = Kuriyama K, Sze PY | title = Blood-brain barrier to H3-gamma-aminobutyric acid in normal and amino oxyacetic acid-treated animals | journal = Neuropharmacology | volume = 10 | issue = 1 | pages = 103–08 | date = January 1971 | pmid = 5569303 | doi = 10.1016/0028-3908(71)90013-X }}</ref> | |||
| Then someone needs to stay with the patient in order to keep monitoring pulse and breathing rate. Finally, someone must stay with the patient until he fully wakes up. This is important, because people tend to become conscious enough to roll onto their backs just before they start to vomit again, but often while they are still too deeply unconscious to protect their own airway. This makes the period while people are waking up particularly dangerous. | |||
| GHB induces the accumulation of either a derivative of ] or tryptophan itself in the extracellular space, possibly by increasing tryptophan transport across the blood–brain barrier.<ref name=":0">{{cite journal | vauthors = Gobaille S, Schleef C, Hechler V, Viry S, Aunis D, Maitre M | title = Gamma-hydroxybutyrate increases tryptophan availability and potentiates serotonin turnover in rat brain | journal = Life Sciences | volume = 70 | issue = 18 | pages = 2101–2112 | date = March 2002 | pmid = 12002803 | doi = 10.1016/s0024-3205(01)01526-0 }}</ref> The blood content of certain neutral amino-acids, including tryptophan, is also increased by peripheral GHB administration. GHB-induced stimulation of tissue ] turnover may be due to an increase in tryptophan transport to the brain and in its uptake by serotonergic cells.<ref name=":0" /><ref>{{cite journal | vauthors = Hedner T, Lundborg P | title = Effect of gammahydroxybutyric acid on serotonin synthesis, concentration and metabolism in the developing rat brain | journal = Journal of Neural Transmission | volume = 57 | issue = 1–2 | pages = 39–48 | date = 1983 | pmid = 6194255 | doi = 10.1007/BF01250046 | s2cid = 9471705 }}</ref> As the serotonergic system may be involved in the regulation of sleep, mood, and anxiety, the stimulation of this system by high doses of GHB may be involved in certain neuropharmacological events induced by GHB administration.<ref>{{cite journal | vauthors = Pardi D, Black J | title = gamma-Hydroxybutyrate/sodium oxybate: neurobiology, and impact on sleep and wakefulness | journal = CNS Drugs | volume = 20 | issue = 12 | pages = 993–1018 | date = 2006 | pmid = 17140279 | doi = 10.2165/00023210-200620120-00004 | s2cid = 72211254 }}</ref> | |||
| Convulsions from GHB can be treated with ] or ], even though these are also CNS depressants they are GABA<sub>A</sub> agonists, whereas GHB is primarily a GABA<sub>B</sub> agonist, so the benzodiazepines do not worsen CNS depression as much as might be expected. | |||
| However, at therapeutic doses, GHB reaches much higher concentrations in the brain and activates GABA<sub>B</sub> receptors, which are primarily responsible for its sedative effects.<ref>{{cite journal | vauthors = Dimitrijevic N, Dzitoyeva S, Satta R, Imbesi M, Yildiz S, Manev H | title = Drosophila GABA(B) receptors are involved in behavioral effects of gamma-hydroxybutyric acid (GHB) | journal = European Journal of Pharmacology | volume = 519 | issue = 3 | pages = 246–252 | date = September 2005 | pmid = 16129424 | doi = 10.1016/j.ejphar.2005.07.016 }}</ref> GHB's sedative effects are blocked by GABA<sub>B</sub> antagonists.<ref>{{cite journal | vauthors = Pistis M, Muntoni AL, Pillolla G, Perra S, Cignarella G, Melis M, Gessa GL | title = Gamma-hydroxybutyric acid (GHB) and the mesoaccumbens reward circuit: evidence for GABA(B) receptor-mediated effects | journal = Neuroscience | volume = 131 | issue = 2 | pages = 465–474 | date = 2005 | pmid = 15708487 | doi = 10.1016/j.neuroscience.2004.11.021 | s2cid = 54342374 }}</ref> | |||
| Most stimulants are not effective at counteracting the unconsciousness from GHB, but intravenous injection of cholinergic drugs such as ], ], and ] can quickly reverse the effects of the GHB and cause rapid awakening; this can be dangerous, however, as these drugs lower the convulsion threshold and so can make convulsions worse. For this reason, these drugs are seldom used in most countries, although, in France and Italy where there is a much longer history of medical use of GHB, physostigmine treatment for GHB overdose is more common. | |||
| The role of the GHB receptor in the behavioural effects induced by GHB is more complex. GHB receptors are densely expressed in many areas of the brain, including the cortex and hippocampus, and these are the receptors that GHB displays the highest affinity for. There has been somewhat limited research into the GHB receptor; however, there is evidence that activation of the GHB receptor in some brain areas results in the release of glutamate, the principal excitatory neurotransmitter.<ref name="nsngtu">{{cite journal | vauthors = Castelli MP, Ferraro L, Mocci I, Carta F, Carai MA, Antonelli T, Tanganelli S, Cignarella G, Gessa GL | title = Selective gamma-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of gamma-hydroxybutyric acid | journal = Journal of Neurochemistry | volume = 87 | issue = 3 | pages = 722–732 | date = November 2003 | pmid = 14535954 | doi = 10.1046/j.1471-4159.2003.02037.x | s2cid = 82175813 }}</ref><ref name=":1">{{cite journal | vauthors = Kamal RM, van Noorden MS, Franzek E, Dijkstra BA, Loonen AJ, De Jong CA | title = The Neurobiological Mechanisms of Gamma-Hydroxybutyrate Dependence and Withdrawal and Their Clinical Relevance: A Review | language = english | journal = Neuropsychobiology | volume = 73 | issue = 2 | pages = 65–80 | date = 2016 | pmid = 27003176 | doi = 10.1159/000443173 | s2cid = 33389634 | doi-access = free | hdl = 2066/158441 | hdl-access = free }}</ref> Drugs that selectively activate the GHB receptor cause ] in high doses, as do GHB and GABA<sub>B</sub> agonists.<ref>{{cite journal | vauthors = Banerjee PK, Snead OC | title = Presynaptic gamma-hydroxybutyric acid (GHB) and gamma-aminobutyric acidB (GABAB) receptor-mediated release of GABA and glutamate (GLU) in rat thalamic ventrobasal nucleus (VB): a possible mechanism for the generation of absence-like seizures induced by GHB | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 273 | issue = 3 | pages = 1534–1543 | date = June 1995 | pmid = 7791129 }}</ref> | |||
| The best treatment of a more serious GHB overdose is co-administration of lorazepam with physostigmine, and the dose of both drugs must be carefully titrated to avoid worsening either the CNS depression or the convulsions. Overdoses with larger quantities of GHB or more particularly GBL (generally 10,000mg or more) can stop both heart and breathing. | |||
| Activation of both the GHB receptor and GABA<sub>B</sub> is responsible for the addictive profile of GHB. GHB's effect on dopamine release is biphasic.<ref>{{cite journal | vauthors = Hechler V, Gobaille S, Bourguignon JJ, Maitre M | title = Extracellular events induced by gamma-hydroxybutyrate in striatum: a microdialysis study | journal = Journal of Neurochemistry | volume = 56 | issue = 3 | pages = 938–944 | date = March 1991 | pmid = 1847191 | doi = 10.1111/j.1471-4159.1991.tb02012.x | s2cid = 86392963 }}</ref><ref name=":1" /> Low concentrations stimulate dopamine release via the GHB receptor.<ref>{{cite journal | vauthors = Maitre M, Hechler V, Vayer P, Gobaille S, Cash CD, Schmitt M, Bourguignon JJ | title = A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 255 | issue = 2 | pages = 657–663 | date = November 1990 | pmid = 2173754 }}</ref><ref>{{cite journal | vauthors = Míguez I, Aldegunde M, Duran R, Veira JA | title = Effect of low doses of gamma-hydroxybutyric acid on serotonin, noradrenaline, and dopamine concentrations in rat brain areas | journal = Neurochemical Research | volume = 13 | issue = 6 | pages = 531–533 | date = June 1988 | pmid = 2457177 | doi = 10.1007/BF00973292 | s2cid = 27073926 }}</ref> Higher concentrations inhibit dopamine release via GABA<sub>B</sub> receptors as do other GABA<sub>B</sub> agonists such as ] and ].<ref>{{cite journal | vauthors = Smolders I, De Klippel N, Sarre S, Ebinger G, Michotte Y | title = Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat | journal = European Journal of Pharmacology | volume = 284 | issue = 1–2 | pages = 83–91 | date = September 1995 | pmid = 8549640 | doi = 10.1016/0014-2999(95)00369-V }}</ref> After an initial phase of inhibition, dopamine release is then increased via the GHB receptor. Both the inhibition and increase of dopamine release by GHB are inhibited by opioid antagonists such as ] and ]. ] may play a role in the inhibition of dopamine release via ]s.<ref>{{cite journal | vauthors = Mamelak M | title = Gammahydroxybutyrate: an endogenous regulator of energy metabolism | journal = Neuroscience and Biobehavioral Reviews | volume = 13 | issue = 4 | pages = 187–198 | year = 1989 | pmid = 2691926 | doi = 10.1016/S0149-7634(89)80053-3 | s2cid = 20217078 }}</ref> | |||
| It can be very dangerous to look after someone who is unconscious as a result of drug overdose if the attending party does not have proper medical training. When an individual presents with a suspected GHB overdose and is unconscious, the first priority should be to check their pulse and breathing. If the patient is taking less than 8 breaths per minute, and if his/her pulse is less than 60 a minute (both numbers are for adults), then the appropriate course of action is to start ] and call an ambulance. | |||
| This explains the paradoxical mix of sedative and stimulatory properties of GHB,<ref>{{cite journal | vauthors = Oliveto A, Gentry WB, Pruzinsky R, Gonsai K, Kosten TR, Martell B, Poling J | title = Behavioral effects of gamma-hydroxybutyrate in humans | journal = Behavioural Pharmacology | volume = 21 | issue = 4 | pages = 332–342 | date = July 2010 | pmid = 20526195 | pmc = 2911496 | doi = 10.1097/FBP.0b013e32833b3397 }}</ref> as well as the so-called "rebound" effect, experienced by individuals using GHB as a sleeping agent, wherein they awake suddenly after several hours of GHB-induced deep sleep. That is to say that, over time, the concentration of GHB in the system decreases below the threshold for significant GABA<sub>B</sub> receptor activation and activates predominantly the GHB receptor, leading to wakefulness. | |||
| A newer synthetic drug ], which acts as a selective GABA<sub>B</sub> antagonist, quickly reverses GHB overdose in mice.<ref name="mousetreatment">{{cite journal | author = Carai, M.A.M. | coauthors = Colombo, G.; Gessa, G.L. | year = 2005 | title = Resuscitative Effect of a γ-Aminobutyric Acid B Receptor Antagonist on γ-Hydroxybutyric Acid Mortality in Mice | journal = Annals of Emergency Medicine | volume = 45 | issue = 6 | pages = 614-619 | doi = 10.1016/j.annemergmed.2004.12.013 }}</ref> However this treatment has yet to be tried in humans, and it is unlikely that it will be researched for this purpose in humans due to the illegal and unethical nature of clinical trials of GHB, and the lack of medical indemnity coverage inherent in using an untested treatment for a life-threatening overdose. | |||
| Recently, analogs of GHB, such as ] (UMB68) have been synthesised and tested on animals, in order to gain a better understanding of GHB's mode of action.<ref>{{cite journal | vauthors = Wu H, Zink N, Carter LP, Mehta AK, Hernandez RJ, Ticku MK, Lamb R, France CP, Coop A | title = A tertiary alcohol analog of gamma-hydroxybutyric acid as a specific gamma-hydroxybutyric acid receptor ligand | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 305 | issue = 2 | pages = 675–79 | date = May 2003 | pmid = 12606613 | doi = 10.1124/jpet.102.046797 | s2cid = 86191608 }}</ref> Analogues of GHB such as 3-methyl-GHB, ], and 4-phenyl-GHB have been shown to produce similar effects to GHB in some animal studies, but these compounds are even less well researched than GHB itself. Of these analogues, only 4-methyl-GHB (γ-hydroxyvaleric acid, GHV) and a prodrug form ] (GVL) have been reported as drugs of abuse in humans, and on the available evidence seem to be less potent but more toxic than GHB, with a particular tendency to cause nausea and vomiting. | |||
| == Addiction == | |||
| GHB can be physically addictive and may result in ]. Physical dependence develops when GHB is taken on a regular basis (i.e., every 2-4 hours for multiple consecutive days or weeks). ] effects may include ], restlessness, ], ]s, sweating, loss of appetite, edginess, ], chest pain and tightness, muscle and bone aches, sensitivity to external stimuli (sound, light, touch), ], and mental dullness. These side-effects will subside after 2 - 21 days, depending on frequency of usage and the size of the doses used. In particularly severe cases, withdrawal from GHB may cause symptoms similar to acute withdrawal from alcohol or barbiturates (]) and can cause convulsions and hallucinations. | |||
| Other prodrug ester forms of GHB have also rarely been encountered by law enforcement, including 1,4-butanediol diacetate (BDDA/DABD), methyl-4-acetoxybutanoate (MAB), and ] (EAB),<ref name="auto"/> but these are, in general, covered by analogue laws in jurisdictions where GHB is illegal, and little is known about them beyond their delayed onset and longer duration of action. The intermediate compound ] (GHBAL) is also a prodrug for GHB; however, as with all ] aldehydes this compound is caustic and is strong-smelling and foul-tasting; actual use of this compound as an intoxicant is likely to be unpleasant and result in severe nausea and vomiting. | |||
| Although there have been reported fatalities due to GHB withdrawal, reports are inconclusive and further research is needed. Unlike ], there is no firm data that chronic use of GHB causes permanent damage to the body. In rats, no organ or brain damage was observed after chronic administration of GBL (a precursor to GHB).<ref>{{Citation | |||
| | author = National Toxicology Program | |||
| | title =Toxicology and Carcinogenesis Studies of g-Butyrolactone (CAS No. 96-48-0) in F344/N Rats and B6C3F1 Mice (Gavage Studies) | |||
| | year = March 1992 | |||
| | pages = | |||
| | place = | |||
| | work = NTP Study Reports | |||
| | publisher = Department of Health and Human Services, NIH | |||
| | url = http://ntp.niehs.nih.gov/index.cfm?objectid=07097962-0CEC-4EFA-62E349AF22EB2E9D}}</ref> | |||
| ] | |||
| == Legal status == | |||
| Only a minor portion (1–5%) of the administered GHB dose is excreted unchanged in the urine. Studies have shown that the maximum concentration of GHB in urine appears within 1 hour and rapidly declines thereafter.<ref name="auto" /> The vast majority (95–98%) undergoes extensive metabolism in the liver. GHB is broken down through a series of enzymatic pathways. The primary route involves conversion to succinic semialdehyde (SSA) by either GHB dehydrogenase (ADH) or GHB transhydrogenase. SSA is further oxidized by succinic semialdehyde dehydrogenase (SSADH) to ], which enters the ] and is ultimately converted into carbon dioxide and water.<ref name="auto" /><ref name="pmid33417072">{{cite journal |vauthors=Felmlee MA, Morse BL, Morris ME |title=γ-Hydroxybutyric Acid: Pharmacokinetics, Pharmacodynamics, and Toxicology |journal=The AAPS Journal |volume=23 |issue=1 |pages=22 |date=January 2021 |pmid=33417072 |pmc=8098080 |doi=10.1208/s12248-020-00543-z}}</ref><ref name="pmid31981617">{{cite journal |vauthors=Taxon ES, Halbers LP, Parsons SM |title=Kinetics aspects of Gamma-hydroxybutyrate dehydrogenase |journal= Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics|volume=1868 |issue=5 |pages=140376 |date=May 2020 |pmid=31981617 |doi=10.1016/j.bbapap.2020.140376|doi-access=free }}</ref><ref name="pmid27003176">{{cite journal |vauthors=Kamal RM, van Noorden MS, Franzek E, Dijkstra BA, Loonen AJ, De Jong CA |title=The Neurobiological Mechanisms of Gamma-Hydroxybutyrate Dependence and Withdrawal and Their Clinical Relevance: A Review |journal=Neuropsychobiology |volume=73 |issue=2 |pages=65–80 |date=March 2016 |pmid=27003176 |doi=10.1159/000443173|hdl=2066/158441 |hdl-access=free }}</ref> | |||
| In ], GHB is regulated under Schedule 1 of ] Chapter 134 ''Dangerous Drugs Ordinance''. It can only be used legally by health professionals and for university research purposes. The substance can be given by pharmacists under a prescription. Anyone who supplies the substance without prescription can be fined $10000 (HKD). The penalty for trafficking or manufacturing the substance is a $5,000,000 (]) fine and life imprisonment. Possession of the substance for consumption without license from the Department of Health is illegal with a $1,000,000 (HKD) fine and/or 7 years of jail time. | |||
| Both of the metabolic breakdown pathways shown for GHB can run in either direction, depending on the concentrations of the substances involved, so the body can make its own GHB either from GABA or from succinic semialdehyde. Under normal physiological conditions, the concentration of GHB in the body is rather low, and the pathways would run in the reverse direction to what is shown here to produce endogenous GHB. However, when GHB is consumed for ] or health promotion purposes, its concentration in the body is much higher than normal, which changes the enzyme kinetics so that these pathways operate to metabolise GHB rather than producing it. | |||
| In the United States it was placed on Schedule I of the ] in March 2000 although when sold as ] it is considered Schedule III, making it the only drug to simultaneously occupy two different schedules.<ref></ref><ref name="erowidGHBlaw" /> On ], ], the ] placed GHB in Schedule IV of the 1971 ].<ref></ref> In the UK it was made a class C drug in June 2003. | |||
| ==History== | |||
| In New Zealand and Australia, GHB, 1,4-B and GBL are all Class B illegal drugs, along with any possible esters, ethers and aldehydes. GABA itself is also listed as an illegal drug in these jurisdictions which seems unusual given its failure to cross the blood-brain barrier, but there was a perception among legislators that all known analogues should be covered as far as this was possible. Attempts to circumvent the illegal status of GHB have led to the sale of derivatives such as 4-methyl-GHB (gamma-hydroxyvaleric acid, GHV) and its prodrug form gamma-valerolactone (GVL), but these are also covered under the law by virtue of their being "substantially similar" to GHB or GBL and; so importation, sale, possession and use of these compounds is also considered to be illegal. | |||
| ] worked on this chemical family and published work on it in 1874.<ref>{{cite book| vauthors = Lewis DE |title=Early Russian organic chemists and their legacy|date=2012|publisher=Springer|isbn=978-3642282195|chapter=Section 4.4.3 Aleksandr Mikhailovich Zaitsev|page=79}}</ref><ref>{{Cite journal | year = 1874 | title = Über die Reduction des Succinylchlorids | journal = ] | volume = 171 | pages = 258–90 | language = de | doi = 10.1002/jlac.18741710216 | issue = 2 | vauthors = Saytzeff A | url = https://zenodo.org/record/1427333 | access-date = 30 June 2019 | archive-date = 4 March 2021 | archive-url = https://web.archive.org/web/20210304115852/https://zenodo.org/record/1427333 | url-status = live }}</ref> The first extended research into GHB and its use in humans was conducted in the early 1960s by ] to use in studying the neurotransmitter GABA.<ref name="WHO">{{cite web|title=Critical review of gamma-hydroxybutyric acid (GHB)|url=https://www.who.int/medicines/areas/quality_safety/4.1GHBcritical_review.pdf |archive-url=https://web.archive.org/web/20140910002840/http://www.who.int/medicines/areas/quality_safety/4.1GHBcritical_review.pdf |archive-date=10 September 2014 |url-status=live|date=2012}}</ref>{{rp|11–12}}<ref>{{cite journal | vauthors = Laborit H, Jouany JM, Gerard J, Fabiani F | title = | language = fr | journal = Agressologie | volume = 1 | pages = 397–406 | date = October 1960 | pmid = 13758011 }}</ref> It was studied in a range of uses including obstetric surgery and during childbirth and as an anxiolytic; there were anecdotal reports of it having antidepressant and aphrodisiac effects as well.<ref name=WHO/>{{rp|27}} It was also studied as an intravenous ] agent and was marketed for that purpose starting in 1964 in Europe but it was not widely adopted as it caused seizures; as of 2006 that use was still authorized in France and Italy but not widely used.<ref name=WHO/>{{rp|27–28}} It was also studied to treat alcohol addiction; while the evidence for this use is weak;<ref name=WHO/>{{rp|28–29}} however, sodium oxybate is marketed for this use in Italy.<ref name="AlcoverLabel">{{cite web|title=Alcover: Riassunto delle Caratteristiche del Prodotto|url=https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000223_027751_RCP.pdf&retry=0&sys=m0b1l3|date=31 March 2017|publisher=Agenzia Italiana del Farmaco|access-date=16 April 2018|archive-date=6 August 2021|archive-url=https://web.archive.org/web/20210806054116/https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000223_027751_RCP.pdf&retry=0&sys=m0b1l3|url-status=dead}} {{Webarchive|url=https://web.archive.org/web/20210304132532/https://farmaci.agenziafarmaco.gov.it/bancadatifarmaci/farmaco?farmaco=027751 |date=4 March 2021 }}</ref> | |||
| GHB and sodium oxybate were also studied for use in ] from the 1960s onwards.<ref name=WHO/>{{rp|28}} | |||
| == GHB in popular culture == | |||
| {{Trivia|date=December 2007}} | |||
| In the "]" episode of '']'', one of the victims, an 18-year-old boy, is found to have died from GHB intoxication. GHB is also named as "the date-rape drug" by one of the investigators, who also relates the information that males often take the drug to get high. In the matter of the GHB-related death, however, the victim had received the GHB without noticing it. | |||
| In May 1990 GHB was introduced as a ] and was marketed to body builders, for help with weight control and as a sleep aid, and as a "replacement" for ], which was removed from the market in November 1989 when batches contaminated with trace impurities<ref>{{cite journal | vauthors = Ito J, Hosaki Y, Torigoe Y, Sakimoto K | title = Identification of substances formed by decomposition of peak E substance in tryptophan | journal = Food and Chemical Toxicology | volume = 30 | issue = 1 | pages = 71–81 | date = January 1992 | pmid = 1544609 | doi = 10.1016/0278-6915(92)90139-C }}</ref> were found to cause ], although eosinophilia–myalgia syndrome is also tied to tryptophan overload.<ref name="pmid16307217">{{cite journal | vauthors = Smith MJ, Garrett RH | title = A heretofore undisclosed crux of eosinophilia-myalgia syndrome: compromised histamine degradation | journal = Inflammation Research | volume = 54 | issue = 11 | pages = 435–50 | date = November 2005 | pmid = 16307217 | doi = 10.1007/s00011-005-1380-7 | s2cid = 7785345 }}</ref> In 2001 tryptophan supplement sales were allowed to resume, and in 2005 the FDA ban on tryptophan supplement importation was lifted.<ref>{{cite book | vauthors = Kapalka GM |title=Nutritional and herbal therapies for children and adolescents : a handbook for mental health clinicians |date=2010 |publisher=Elsevier/AP |isbn=978-0-12-374927-7}}</ref> By November 1989 57 cases of illness caused by the GHB supplements had been reported to the ], with people having taken up to three teaspoons of GHB; there were no deaths but nine people needed care in an ].<ref name="MMWR">{{cite journal | title = Multistate outbreak of poisonings associated with illicit use of gamma hydroxy butyrate | journal = MMWR. Morbidity and Mortality Weekly Report | volume = 39 | issue = 47 | pages = 861–63 | date = November 1990 | pmid = 2122223 | url = https://www.cdc.gov/mmwr/preview/mmwrhtml/00001847.htm | author1 = Centers for Disease Control (CDC) | access-date = 16 April 2018 | archive-date = 6 June 2021 | archive-url = https://web.archive.org/web/20210606183318/https://www.cdc.gov/mmwr/preview/mmwrhtml/00001847.htm | url-status = live }}</ref><ref>{{cite journal | vauthors = Dyer JE | title = gamma-Hydroxybutyrate: a health-food product producing coma and seizurelike activity | journal = The American Journal of Emergency Medicine | volume = 9 | issue = 4 | pages = 321–24 | date = July 1991 | pmid = 2054002 | doi = 10.1016/0735-6757(91)90050-t }}</ref> The FDA issued a warning in November 1990 that sale of GHB was illegal.<ref name=MMWR/> GHB continued to be manufactured and sold illegally and it and analogs were adopted as a ] and came to be used as a ], and the DEA made seizures and the FDA reissued warnings several times throughout the 1990s.<ref>{{cite book|author1=Institute of Medicine|author2=National Research Council (US) Committee on the Framework for Evaluating the Safety of Dietary Supplements|title=Proposed Framework for Evaluating the Safety of Dietary Supplements: For Comment.|date=2002|publisher=National Academies Press (US)|chapter-url=https://www.ncbi.nlm.nih.gov/books/NBK220875/|chapter=Appendix D: Table of Food and Drug Administration Actions on Dietary Supplements|access-date=16 April 2018|archive-date=29 August 2021|archive-url=https://web.archive.org/web/20210829063758/https://www.ncbi.nlm.nih.gov/books/NBK220875/|url-status=live}}</ref><ref>{{cite journal|title=GHB: A Club Drug To Watch|journal=Substance Abuse Treatment Advisory|date=November 2002|volume=2|issue=1|url=https://store.samhsa.gov/shin/content/SMA03-3766/SMA03-3766.pdf|access-date=16 April 2018|archive-url=https://web.archive.org/web/20170801221141/https://store.samhsa.gov/shin/content/SMA03-3766/SMA03-3766.pdf|archive-date=1 August 2017|url-status=dead}}</ref><ref>{{cite journal | vauthors = Mason PE, Kerns WP | s2cid = 13325306 | title = Gamma hydroxybutyric acid (GHB) intoxication | journal = Academic Emergency Medicine | volume = 9 | issue = 7 | pages = 730–79 | date = July 2002 | pmid = 12093716 | doi = 10.1197/aemj.9.7.730 | doi-access = free }}</ref> | |||
| In the '']'' episode "]" GHB was used to drug Sarah McGee, Special Agent Timothy McGee's sister, into thinking that she committed a murder as a cover-up. A classmate, whom she despises, drugged her by adding it to her peanut butter. | |||
| At the same time, research on the use of GHB in the form of sodium oxybate had formalized, as a company called Orphan Medical had filed an investigational new drug application and was running clinical trials with the intention of gaining regulatory approval for use to treat narcolepsy.<ref name=WHO/>{{rp|18–25, 28}}<ref>{{cite web|title=Transcript: FDA Peripheral and Central Nervous System Drugs Advisory Committee Meeting|url=https://www.fda.gov/ohrms/dockets/ac/01/transcripts/3754t1.txt|publisher=FDA|date=6 June 2001|access-date=16 April 2018|archive-date=16 May 2017|archive-url=https://web.archive.org/web/20170516063446/https://www.fda.gov/ohrms/dockets/ac/01/transcripts/3754t1.txt|url-status=dead}}</ref>{{rp|10}} | |||
| In the pilot episode of '']'', Veronica tells us she was raped at a party when she was sixteen. At the end of episode 20, "M.A.D.", of season 1, Veronica discovers that she had been given GHB at the party and investigates that night in the following episode, "A Trip to the Dentist". This party is referenced throughout season one and is a major conflict for the character, which was revisited in the final episode of season 2 when she finally learned exactly who she was raped by that night. | |||
| A popular children's toy, ] (also known as Aqua Dots, in the United States), produced by Melbourne company Moose, was banned in Australia in early November 2007 when it was discovered that 1,4-butanediol (1,4-B), which is ] into GHB, had been substituted for the non-toxic plasticiser ] in the bead manufacturing process. Three young children were hospitalized as a result of ingesting a large number of the beads, and the toy was recalled.<ref>{{cite news | vauthors = Perry M, Pomfret J, Crabb RP | title = Australia bans China-made toy on toxic drug risk | date = 7 November 2007 | work = Reuters | url = https://www.reuters.com/article/worldNews/idUSSYD2129620071107 | access-date = 2 July 2017 | archive-date = 5 April 2023 | archive-url = https://web.archive.org/web/20230405014132/https://www.reuters.com/article/worldNews/idUSSYD2129620071107 | url-status = live }}</ref> | |||
| The current season 3 of '']'' centers around the main character's freshman year at Hearst College, where she investigates a string of GHB-related rapes on campus. An episode of '']'' involves a woman who is drugged with GHB by a vengeful colleague in hopes that the woman will be raped. In the '']'' episode "Fools for Love," one of the victim's deaths is attributed to choking on vomit from a GHB overdose administered by her rapist/killer. | |||
| <includeonly></includeonly> | |||
| The television series '']'' featured GHB in a multi-episode story during the conclusion of season 4 and the beginning of season 5. The president's daughter consumes GHB that had been slipped into an alcoholic beverage without her knowledge, and, as she feels the effects of the drug, her boyfriend implies that she has consumed ecstasy. Later, she is kidnapped from a nightclub bathroom while barely or not conscious, setting off a massive manhunt. The president is told later by an FBI agent that the drug is created by mixing degreasing solvent and drain cleaner, and he finishes the agent's sentence by acknowledging he is aware the drug is known as a date-rape drug. | |||
| == Legal status == | |||
| The French activist and psychoanalytic theorist ] used GHB recreationally in the early 1970s, see '']'' (2006:308,326) | |||
| ] | |||
| In the United States, GHB was placed on ] of the Controlled Substances Act in March 2000. However, used in sodium oxybate under an IND or NDA from the US FDA, it is considered a Schedule III substance but with Schedule I trafficking penalties, one of several drugs that are listed in multiple schedules.<ref name="DEA">{{cite web|title=2000 – Addition of Gamma-Hydroxybutyric Acid to Schedule I|url=https://www.deadiversion.usdoj.gov/fed_regs/rules/2000/fr0313.htm|publisher=US Department of Justice via the Federal Register|date=13 March 2000|access-date=16 April 2018|archive-date=1 May 2021|archive-url=https://web.archive.org/web/20210501154805/https://www.deadiversion.usdoj.gov/fed_regs/rules/2000/fr0313.htm|url-status=dead}}</ref><ref>{{cite web|title=William J. Clinton: Statement on Signing the Hillory J. Farias and Samantha Reid Date-Rape Drug Prohibition Act of 2000|url=http://www.presidency.ucsb.edu/ws/index.php?pid=58098|date=18 February 2000|access-date=16 April 2018|archive-date=13 September 2018|archive-url=https://web.archive.org/web/20180913115803/http://www.presidency.ucsb.edu/ws/index.php?pid=58098|url-status=live}}</ref> | |||
| Near the end of the second season of the television series '']'', leading character Amy Abbott is pressured into consuming a low dose of GHB dissolved in water with her then-boyfriend, allegedly-recovered addict Tommy Callahan. In a misguided effort to keep her from accidentally overdosing, he consumes most of the drugs before giving her the remainder, thus overdosing himself. Upon realizing he was unresponsive, Amy called her doctor father to come to his rescue, and, while they succeeded in saving Tommy's life, this encounter with the controlled substance ended their relationship. | |||
| On 20 March 2001, the UN ] placed GHB in Schedule IV of the 1971 ].<ref name="Whitehouse factsheet">{{Cite web|url=http://www.whitehousedrugpolicy.gov/publications/factsht/gamma/ |title=Gamma Hydroxybutyrate (GHB) |year=2002 |archive-url=https://web.archive.org/web/20051231122054/http://www.whitehousedrugpolicy.gov/publications/factsht/gamma/ |archive-date=31 December 2005 |access-date=20 November 2016 |url-status=dead }}</ref> | |||
| An episode of the fifth series of '']'' shows Ros using a refined form of the drug to incapacitate targets for intelligence gathering. | |||
| In the UK GHB was made a class C drug in June 2003. In October 2013 the ] recommended upgrading it from schedule IV to schedule II in line with UN recommendations. Their report concluded that the minimal use of Xyrem in the UK meant that prescribers would be minimally inconvenienced by the rescheduling.<ref name="ACMD scheduling recommendation">{{cite web | url = https://www.gov.uk/government/publications/acmd-advice-on-the-scheduling-of-ghb | title = ACMD advice on the scheduling of GHB | access-date = 23 October 2013 | vauthors = Iversen L | date = 3 October 2013 | format = PDF | publisher = UK Home Office | archive-date = 13 August 2014 | archive-url = https://web.archive.org/web/20140813235435/https://www.gov.uk/government/publications/acmd-advice-on-the-scheduling-of-ghb | url-status = live }}</ref> This advice was followed and GHB was moved to schedule 2 on 7 January 2015.<ref>{{Cite web |url=https://www.gov.uk/government/publications/circular-0012015-a-change-to-the-misuse-of-drugs-act-1971-control-of-ah-7921-lsd-related-compounds-tryptamines-and-rescheduling-of-ghb/circular-0012015-a-change-to-the-misuse-of-drugs-act-1971-control-of-ah-7921-lsd-related-compounds-tryptamines-and-rescheduling-of-ghb |title=Circular 001/2015: A Change to the Misuse of Drugs Act 1971: control of AH-7921, LSD–related compounds, tryptamines, and rescheduling of GHB |year=2015 |publisher=UK Home Office |access-date=8 May 2017 |archive-date=6 April 2023 |archive-url=https://web.archive.org/web/20230406095103/https://www.gov.uk/government/publications/circular-0012015-a-change-to-the-misuse-of-drugs-act-1971-control-of-ah-7921-lsd-related-compounds-tryptamines-and-rescheduling-of-ghb/circular-0012015-a-change-to-the-misuse-of-drugs-act-1971-control-of-ah-7921-lsd-related-compounds-tryptamines-and-rescheduling-of-ghb |url-status=live }}</ref><ref>{{Cite web |url=http://www.legislation.gov.uk/uksi/2014/3277/made |title=The Misuse of Drugs (Amendment No. 3) (England, Wales and Scotland) Regulations 2014 |date=11 December 2014 |publisher=UK Home Office |access-date=8 May 2017 |archive-date=5 August 2023 |archive-url=https://web.archive.org/web/20230805175941/https://www.legislation.gov.uk/uksi/2014/3277/made |url-status=live }}</ref> In April 2022 GHB was changed from class C to class B.<ref>{{cite web | title = The Misuse of Drugs Act 1971 (Amendment) Order 2022 | url = https://www.legislation.gov.uk/uksi/2022/322/note/made | work = www.legislation.gov.uk | access-date = 15 June 2023 | archive-date = 26 July 2023 | archive-url = https://web.archive.org/web/20230726023326/https://www.legislation.gov.uk/uksi/2022/322/note/made | url-status = live }}</ref> | |||
| In the third episode of the American version of "]," the character Ted falls into a coma after being given a large dose of GHB by a date he brings home. | |||
| In Hong Kong, GHB is regulated under Schedule 1 of Hong Kong's Chapter 134 ''Dangerous Drugs Ordinance''. It can only be used legally by health professionals and for university research purposes. The substance can be given by pharmacists under a prescription. Anyone who supplies the substance without prescription can be fined HK$10,000. The penalty for trafficking or manufacturing the substance is a HK$150,000 fine and life imprisonment. Possession of the substance for consumption without license from the Department of Health is illegal with a HK$100,000 fine or five years of jail time. | |||
| In the popular funk song "Party Song" by ], GHB is referenced in a clever line: "A to the B to the C to the D to the E to the F to the GHB." The song also references "Hangin' out with my Georgia Homeboy." | |||
| In Canada, GHB has been a Schedule I controlled substance since 6 November 2012 (the same schedule that contains heroin and cocaine). Prior to that date, it was a Schedule III controlled substance (the same schedule that contains amphetamines and LSD).<ref>{{cite web |title=Controlled Drugs and Substances Act, SC 1996, c 19 |url=https://www.canlii.org/en/ca/laws/stat/sc-1996-c-19/latest/sc-1996-c-19.html#history |publisher=Canadian Legal Information Institute |access-date=25 November 2018 |archive-date=17 June 2019 |archive-url=https://web.archive.org/web/20190617175124/https://www.canlii.org/en/ca/laws/stat/sc-1996-c-19/latest/sc-1996-c-19.html#history |url-status=live }}</ref> | |||
| In the song "What's Going On" by ], found on their album ], GHB is referenced as a ] drug: She takes another sip/ | |||
| But has no clue of the spike from the G to the H to the B/ She wakes up in the morning/ bruised and raped in the street. GHB is referred to in the song ''Shores of California'' by ]: ''And that is why the girl is called a tease / and that is why the guy is called a sleaze / and that's why God made escort agencies / one life to live and mace and GHB.'' | |||
| In New Zealand and Australia, GHB, 1,4-B, and GBL are all Class B illegal drugs, along with any possible esters, ethers, and aldehydes. GABA itself is also listed as an illegal drug in these jurisdictions, which seems unusual given its failure to cross the blood–brain barrier, but there was a perception among legislators that all known analogues should be covered as far as this was possible. Attempts to circumvent the illegal status of GHB have led to the sale of derivatives such as 4-methyl-GHB (γ-hydroxyvaleric acid, GHV) and its prodrug form γ-valerolactone (GVL), but these are also covered under the law by virtue of their being "substantially similar" to GHB or GBL, so importation, sale, possession and use of these compounds is also considered to be illegal. | |||
| On the ] album 'Cyberpunk' (1993), on the song 'Then the Night Comes', GHB is mentioned at 2:30 into the song, as follows...'I take some GHB, I feel love, joy, And wonderful ringing music, Now, I just got to be me'. Following the album's release, he (Idol) almost died of a GHB overdose in 1994. | |||
| In Chile, GHB is a controlled drug under the law {{lang|es|Ley de substancias psicotrópicas y estupefacientes}} (psychotropic substances and narcotics). | |||
| In ''The Anniversary'', by ], GHB is referenced as a date rape drug. | |||
| In Norway<ref name="urlFOR 30 June 1978 nr 08: Forskrift om narkotika m.v. (Narkotikalisten)">{{cite web|url=http://www.lovdata.no/cgi-wift/ldles?doc=/sf/sf/sf-19780630-0008.html|title=FOR 30 June 1978 nr 08: Forskrift om narkotika m.v. (Narkotikalisten)|language=no|access-date=17 August 2008|archive-date=16 October 2014|archive-url=https://web.archive.org/web/20141016174713/http://www.lovdata.no/cgi-wift/ldles?doc=/sf/sf/sf-19780630-0008.html|url-status=live}}</ref> and in Switzerland,<ref name="Kompendium Xyrem"> {{dead link|date=October 2017|bot=InternetArchiveBot|fix-attempted=yes}}</ref> GHB is considered a narcotic and is only available by prescription under the trade name Xyrem (]). | |||
| Sodium oxybate is also used therapeutically in Italy under the brand name Alcover for treatment of ] and dependence.<ref>{{cite journal | vauthors = Haller C, Thai D, Jacob P, Dyer JE | title = GHB urine concentrations after single-dose administration in humans | journal = Journal of Analytical Toxicology | volume = 30 | issue = 6 | pages = 360–64 | year = 2006 | pmid = 16872565 | pmc = 2257868 | doi = 10.1093/jat/30.6.360 }}</ref> | |||
| == See also == | |||
| * ] | |||
| * ] (GHV) | |||
| * ] (GVL) | |||
| * ] (HMB) | |||
| == References == | == References == | ||
| {{ |
{{Reflist}} | ||
| == |
==External links== | ||
| {{Commons category|GHB}} | |||
| * | |||
| * | |||
| * | * | ||
| * ] (also contains information about addiction and dangers) | |||
| * | |||
| * (]) | |||
| * | |||
| * | |||
| * (contains also information about addiction and dangers) | |||
| * (]) | |||
| * | |||
| * | |||
| {{ |
{{Drug use}} | ||
| {{Neurotransmitters}} | |||
| {{Sedatives}} | |||
| {{Euphoriants}} | |||
| {{GHBergics}} | |||
| {{GABAergics}} | |||
| {{Authority control}} | |||
| {{DEFAULTSORT:Hydroxybutyric Acid, γ-}} | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 15:14, 14 December 2024
Chemical compound "GHB" redirects here. For other uses, see GHB (disambiguation).Pharmaceutical compound
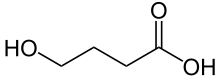 | |
 | |
| Clinical data | |
|---|---|
| Other names |
|
| Addiction liability | High |
| Routes of administration | By mouth, intravenous |
| Drug class | GABA analogue, GHB receptor agonists—GABA receptor agonist; Psycholeptic;
Depressant; Hypnotic Sedative |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25% (oral) |
| Metabolism | 95–98%, mainly liver, also in blood and tissues |
| Onset of action | Within 5–15 minutes |
| Elimination half-life | 30–60 minutes |
| Excretion | 1–5%, kidney |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.218.519 |
| Chemical and physical data | |
| Formula | C4H8O3 |
| Molar mass | 104.105 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
γ-Hydroxybutyric acid, also known as gamma-hydroxybutyric acid, GHB, or 4-hydroxybutanoic acid, is a naturally occurring neurotransmitter and a depressant drug. It is a precursor to GABA, glutamate, and glycine in certain brain areas. It acts on the GHB receptor and is a weak agonist at the GABAB receptor. GHB has been used in the medical setting as a general anesthetic and as treatment for cataplexy, narcolepsy, and alcoholism. The substance is also used illicitly for various reasons, including as a performance-enhancing drug, date rape drug, and as a recreational drug.
It is commonly used in the form of a salt, such as sodium γ-hydroxybutyrate (NaGHB, sodium oxybate, or Xyrem) or potassium γ-hydroxybutyrate (KGHB, potassium oxybate). GHB is also produced as a result of fermentation, and is found in small quantities in some beers and wines, beef, and small citrus fruits.
Succinic semialdehyde dehydrogenase deficiency is a disease that causes GHB to accumulate in the blood.
Medical use
Main article: Sodium oxybateGHB is used for medical purposes in the treatment of narcolepsy and, more rarely, alcohol dependence, although there remains uncertainty about its efficacy relative to other pharmacotherapies for alcohol dependence. The authors of a 2010 Cochrane review concluded that "GHB appears better than NTX and disulfiram in maintaining abstinence and preventing craving in the medium term (3 to 12 months)". It is sometimes used off-label for the treatment of fibromyalgia. GHB is the active ingredient of the prescription medication sodium oxybate (Xyrem). Sodium oxybate is approved by the U.S. Food and Drug Administration for the treatment of cataplexy associated with narcolepsy and excessive daytime sleepiness (EDS) associated with narcolepsy.
GHB has been shown to reliably increase slow-wave sleep and decrease the tendency for REM sleep in modified multiple sleep latency tests.
The FDA-approved labeling for sodium oxybate suggests no evidence GHB has teratogenic, carcinogenic or hepatotoxic properties. Its favorable safety profile relative to ethanol may explain why GHB continues to be investigated as a candidate for alcohol substitution.
Recreational use
GHB is a central nervous system depressant used as an intoxicant. It has many street names. Its effects have been described as comparable with ethanol (alcohol) and MDMA use, such as euphoria, disinhibition, enhanced libido and empathogenic states. A review comparing ethanol to GHB concluded that the dangers of the two drugs were similar. At higher doses, GHB may induce nausea, dizziness, drowsiness, agitation, visual disturbances, depressed breathing, amnesia, unconsciousness, and death. One potential cause of death from GHB consumption is polydrug toxicity. Co-administration with other CNS depressants such as alcohol or benzodiazepines can result in an additive effect (potentiation), as they all bind to gamma-aminobutyric acid (or "GABA") receptor sites. The effects of GHB can last from 1.5 to 4 hours, or longer if large doses have been consumed. Consuming GHB with alcohol can cause respiratory arrest and vomiting in combination with unarousable sleep, which can lead to death.
Recreational doses of 1–2 g generally provide a feeling of euphoria, and larger doses create deleterious effects such as reduced motor function and drowsiness. The sodium salt of GHB has a salty taste. Other salt forms such as calcium GHB and magnesium GHB have also been reported, but the sodium salt is by far the most common.
Some prodrugs, such as γ-butyrolactone (GBL), convert to GHB in the stomach and bloodstream. Other prodrugs exist, such as 1,4-butanediol (1,4-B). GBL and 1,4-B are normally found as pure liquids, but they can be mixed with other more harmful solvents when intended for industrial use (e.g. as paint stripper or varnish thinner).
GHB can be manufactured with little knowledge of chemistry, as it involves the mixing of its two precursors, GBL and an alkali hydroxide such as sodium hydroxide, to form the GHB salt. Due to the ease of manufacture and the availability of its precursors, it is not usually produced in illicit laboratories like other synthetic drugs, but in private homes by low-level producers.
GHB is colourless and odourless.
Party use
GHB has been used as a club drug, apparently starting in the 1990s, as small doses of GHB can act as a euphoriant and are believed to be aphrodisiac. Slang terms for GHB include liquid ecstasy, lollipops, liquid X or liquid E due to its tendency to produce euphoria and sociability and its use in the dance party scene.
Sports and athletics
Some athletes have used GHB or its analogs because of being marketed as anabolic agents, although there is no evidence that it builds muscle or improves performance.
Usage as a date-rape drug

GHB became known to the general public as a date-rape drug by the late 1990s. GHB is colourless and odorless and has been described as "very easy to add to drinks". When consumed, the victim will quickly feel groggy and sleepy and may become unconscious. Upon recovery they may have an impaired ability to recall events that have occurred during the period of intoxication. In these situations evidence and the identification of the perpetrator of the rape is often difficult.
It is also difficult to establish how often GHB is used to facilitate rape as it is difficult to detect in a urine sample after a day, and many victims may only recall the rape some time after its occurrence; however, a 2006 study suggested that there was "no evidence to suggest widespread date rape drug use" in the UK, and that less than 2% of cases involved GHB, while 17% involved cocaine, and a survey in the Netherlands published in 2010 found that the proportion of drug-related rapes where GHB was used appeared to be greatly overestimated by the media. More recently, a study in Western Australia reviewed the pre-hospital context given in medical records around emergency department presentations with analytical confirmation of GHB exposure. This study found that most cases reported daily dosing and subsequent accidental overdose rather than their presentation being associated with date-rape.
There have been several high-profile cases of GHB as a date-rape drug that received national attention in the United States. In early 1999, a 15-year-old girl, Samantha Reid of Rockwood, Michigan, died from GHB poisoning. Reid's death inspired the legislation titled the "Hillory J. Farias and Samantha Reid Date-Rape Drug Prohibition Act of 2000". This is the law that made GHB a Schedule 1 controlled substance. In the United Kingdom, British serial killer Stephen Port administered GHB to his victims by adding it to drinks given to them, raping them, and murdering four of them in his flat in Barking, East London.
GHB can be detected in hair. Hair testing can be a useful tool in court cases or for the victim's own information. Most over-the-counter urine test kits test only for date-rape drugs that are benzodiazepines, which GHB is not. To detect GHB in urine, the sample must be taken within four hours of GHB ingestion, and cannot be tested at home.
Adverse effects
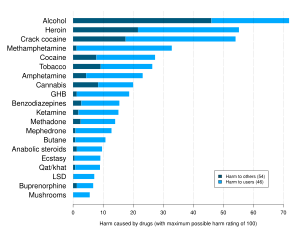
Combination with alcohol
In humans, GHB has been shown to reduce the elimination rate (thus increasing the elimination time) of alcohol. This may explain the respiratory arrest that has been reported after ingestion of both drugs. A review of the details of 194 deaths attributed to or related to GHB over a ten-year period found that most were from respiratory depression caused by interaction with alcohol or other drugs.
Deaths
One publication has investigated 226 deaths attributed to GHB. Of the 226 deaths included, 213 had a cardiorespiratory arrest and 13 had fatal accidents. Seventy-one of these deaths (34%) had no co-intoxicants. Postmortem blood GHB was 18–4400 mg/L (median=347) in deaths negative for co-intoxicants.
One report has suggested that sodium oxybate overdose might be fatal, based on deaths of three patients who had been prescribed the drug. However, for two of the three cases, post-mortem GHB concentrations were 141 and 110 mg/L, which is within the expected range of concentrations for GHB after death, and the third case was a patient with a history of intentional drug overdose. The toxicity of GHB has been an issue in criminal trials, as in the death of Felicia Tang, where the defense argued that death was due to GHB, not murder.
GHB is produced in the body in very small amounts, and blood levels may climb after death to levels in the range of 30–50 mg/L. Levels higher than this are found in GHB deaths. Levels lower than this may be due to GHB or to postmortem endogenous elevations.
Neurotoxicity
In multiple studies, GHB has been found to impair spatial memory, working memory, learning and memory in rats with chronic administration. These effects are associated with decreased NMDA receptor expression in the cerebral cortex and possibly other areas as well. In addition, the neurotoxicity appears to be caused by oxidative stress.
Addiction
Addiction occurs when repeated drug use disrupts the normal balance of brain circuits that control rewards, memory and cognition, ultimately leading to compulsive drug taking.
Rats forced to consume massive doses of GHB will intermittently prefer GHB solution to water.
Withdrawal
GHB has also been associated with a withdrawal syndrome of insomnia, anxiety, and tremor that usually resolves within three to twenty-one days. The withdrawal syndrome can be severe producing acute delirium and may require hospitalization in an intensive care unit for management. Management of GHB dependence involves considering the person's age, comorbidity and the pharmacological pathways of GHB. The mainstay of treatment for severe withdrawal is supportive care and benzodiazepines for control of acute delirium, but larger doses are often required compared to acute delirium of other causes (e.g. > 100 mg/d of diazepam). Baclofen has been suggested as an alternative or adjunct to benzodiazepines based on anecdotal evidence and some animal data. However, there is less experience with the use of baclofen for GHB withdrawal, and additional research in humans is needed. Baclofen was first suggested as an adjunct because benzodiazepines do not affect GABAB receptors and therefore have no cross-tolerance with GHB while baclofen, which works via GABAB receptors, is cross-tolerant with GHB and may be more effective in alleviating withdrawal effects of GHB.
GHB withdrawal is not widely discussed in textbooks and some psychiatrists, general practitioners, and even hospital emergency physicians may not be familiar with this withdrawal syndrome.
Overdose
Overdose of GHB can sometimes be difficult to treat because of its multiple effects on the body. GHB tends to cause rapid unconsciousness at doses above 3500 mg, with single doses over 7000 mg often causing life-threatening respiratory depression, and higher doses still inducing bradycardia and cardiac arrest. Other side-effects include convulsions (especially when combined with stimulants), and nausea/vomiting (especially when combined with alcohol).
The greatest life threat due to GHB overdose (with or without other substances) is respiratory arrest. Other relatively common causes of death due to GHB ingestion include aspiration of vomitus, positional asphyxia, and trauma sustained while intoxicated (e.g., motor vehicle accidents while driving under the influence of GHB). The risk of aspiration pneumonia and positional asphyxia risk can be reduced by laying the patient down in the recovery position. People are most likely to vomit as they become unconscious, and as they wake up. It is important to keep the victim awake and moving; the victim must not be left alone due to the risk of death through vomiting. Frequently the victim will be in a good mood but this does not mean the victim is not in danger. GHB overdose is a medical emergency and immediate assessment in an emergency department is needed.
Convulsions from GHB can be treated with the benzodiazepines diazepam or lorazepam. Even though these benzodiazepines are also CNS depressants, they primarily modulate GABAA receptors whereas GHB is primarily a GABAB receptor agonist, and so do not worsen CNS depression as much as might be expected.
Because of the faster and more complete absorption of GBL relative to GHB, its dose-response curve is steeper, and overdoses of GBL tend to be more dangerous and problematic than overdoses involving only GHB or 1,4-B. Any GHB/GBL overdose is a medical emergency and should be cared for by appropriately trained personnel.
A newer synthetic drug, SCH-50911, which acts as a selective GABAB antagonist, quickly reverses GHB overdose in mice. However, this treatment has yet to be tried in humans, and it is unlikely that it will be researched for this purpose in humans due to the illegal nature of clinical trials of GHB and the lack of medical indemnity coverage inherent in using an untested treatment for a life-threatening overdose.
Detection of use
GHB may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized patients, to provide evidence in an impaired driving, or to assist in a medicolegal death investigation. Blood or plasma GHB concentrations are usually in a range of 50–250 mg/L in persons receiving the drug therapeutically (during general anesthesia), 30–100 mg/L in those arrested for impaired driving, 50–500 mg/L in acutely intoxicated patients and 100–1000 mg/L in victims of fatal overdosage. Urine is often the preferred specimen for routine drug abuse monitoring purposes. Both γ-butyrolactone (GBL) and 1,4-butanediol are converted to GHB in the body.
In January 2016, it was announced scientists had developed a way to detect GHB, among other things, in saliva.
Endogenous production
Cells produce GHB by reduction of succinic semialdehyde via succinic semialdehyde reductase (SSR). This enzyme appears to be induced by cAMP levels, meaning substances that elevate cAMP, such as forskolin and vinpocetine, may increase GHB synthesis and release. Conversely, endogeneous GHB production in those taking valproic acid will be inhibited via inhibition of the conversion from succinic acid semialdehyde to GHB. People with the disorder known as succinic semialdehyde dehydrogenase deficiency, also known as γ-hydroxybutyric aciduria, have elevated levels of GHB in their urine, blood plasma and cerebrospinal fluid.
The precise function of GHB in the body is not clear. It is known, however, that the brain expresses a large number of receptors that are activated by GHB. These receptors are excitatory, however, and therefore not responsible for the sedative effects of GHB; they have been shown to elevate the principal excitatory neurotransmitter, glutamate. The benzamide antipsychotics—amisulpride, nemonapride, etc.—have been shown to bind to these GHB-activated receptors in vivo. Other antipsychotics were tested and were not found to have an affinity for this receptor.
GHB is a precursor to GABA, glutamate, and glycine in certain brain areas.
In spite of its demonstrated neurotoxicity, (see relevant section, above), GHB has neuroprotective properties, and has been found to protect cells from hypoxia.
Natural fermentation by-product
GHB is also produced as a result of fermentation and so is found in small quantities in some beers and wines, in particular fruit wines. The amount found in wine is pharmacologically insignificant and not sufficient to produce psychoactive effects.
Pharmacology
GHB has at least two distinct binding sites in the central nervous system. GHB acts as an agonist at the inhibitory GHB receptor and as a weak agonist at the inhibitory GABAB receptor. GHB is a naturally occurring substance that acts in a similar fashion to some neurotransmitters in the mammalian brain. GHB is probably synthesized from GABA in GABAergic neurons, and released when the neurons fire.
GHB has been found to activate oxytocinergic neurons in the supraoptic nucleus.
If taken orally, GABA itself does not effectively cross the blood–brain barrier.
GHB induces the accumulation of either a derivative of tryptophan or tryptophan itself in the extracellular space, possibly by increasing tryptophan transport across the blood–brain barrier. The blood content of certain neutral amino-acids, including tryptophan, is also increased by peripheral GHB administration. GHB-induced stimulation of tissue serotonin turnover may be due to an increase in tryptophan transport to the brain and in its uptake by serotonergic cells. As the serotonergic system may be involved in the regulation of sleep, mood, and anxiety, the stimulation of this system by high doses of GHB may be involved in certain neuropharmacological events induced by GHB administration.
However, at therapeutic doses, GHB reaches much higher concentrations in the brain and activates GABAB receptors, which are primarily responsible for its sedative effects. GHB's sedative effects are blocked by GABAB antagonists.
The role of the GHB receptor in the behavioural effects induced by GHB is more complex. GHB receptors are densely expressed in many areas of the brain, including the cortex and hippocampus, and these are the receptors that GHB displays the highest affinity for. There has been somewhat limited research into the GHB receptor; however, there is evidence that activation of the GHB receptor in some brain areas results in the release of glutamate, the principal excitatory neurotransmitter. Drugs that selectively activate the GHB receptor cause absence seizures in high doses, as do GHB and GABAB agonists.
Activation of both the GHB receptor and GABAB is responsible for the addictive profile of GHB. GHB's effect on dopamine release is biphasic. Low concentrations stimulate dopamine release via the GHB receptor. Higher concentrations inhibit dopamine release via GABAB receptors as do other GABAB agonists such as baclofen and phenibut. After an initial phase of inhibition, dopamine release is then increased via the GHB receptor. Both the inhibition and increase of dopamine release by GHB are inhibited by opioid antagonists such as naloxone and naltrexone. Dynorphin may play a role in the inhibition of dopamine release via kappa opioid receptors.
This explains the paradoxical mix of sedative and stimulatory properties of GHB, as well as the so-called "rebound" effect, experienced by individuals using GHB as a sleeping agent, wherein they awake suddenly after several hours of GHB-induced deep sleep. That is to say that, over time, the concentration of GHB in the system decreases below the threshold for significant GABAB receptor activation and activates predominantly the GHB receptor, leading to wakefulness.
Recently, analogs of GHB, such as 4-hydroxy-4-methylpentanoic acid (UMB68) have been synthesised and tested on animals, in order to gain a better understanding of GHB's mode of action. Analogues of GHB such as 3-methyl-GHB, 4-methyl-GHB, and 4-phenyl-GHB have been shown to produce similar effects to GHB in some animal studies, but these compounds are even less well researched than GHB itself. Of these analogues, only 4-methyl-GHB (γ-hydroxyvaleric acid, GHV) and a prodrug form γ-valerolactone (GVL) have been reported as drugs of abuse in humans, and on the available evidence seem to be less potent but more toxic than GHB, with a particular tendency to cause nausea and vomiting.
Other prodrug ester forms of GHB have also rarely been encountered by law enforcement, including 1,4-butanediol diacetate (BDDA/DABD), methyl-4-acetoxybutanoate (MAB), and ethyl-4-acetoxybutanoate (EAB), but these are, in general, covered by analogue laws in jurisdictions where GHB is illegal, and little is known about them beyond their delayed onset and longer duration of action. The intermediate compound γ-hydroxybutyraldehyde (GHBAL) is also a prodrug for GHB; however, as with all aliphatic aldehydes this compound is caustic and is strong-smelling and foul-tasting; actual use of this compound as an intoxicant is likely to be unpleasant and result in severe nausea and vomiting.

Only a minor portion (1–5%) of the administered GHB dose is excreted unchanged in the urine. Studies have shown that the maximum concentration of GHB in urine appears within 1 hour and rapidly declines thereafter. The vast majority (95–98%) undergoes extensive metabolism in the liver. GHB is broken down through a series of enzymatic pathways. The primary route involves conversion to succinic semialdehyde (SSA) by either GHB dehydrogenase (ADH) or GHB transhydrogenase. SSA is further oxidized by succinic semialdehyde dehydrogenase (SSADH) to succinic acid, which enters the Krebs cycle and is ultimately converted into carbon dioxide and water.
Both of the metabolic breakdown pathways shown for GHB can run in either direction, depending on the concentrations of the substances involved, so the body can make its own GHB either from GABA or from succinic semialdehyde. Under normal physiological conditions, the concentration of GHB in the body is rather low, and the pathways would run in the reverse direction to what is shown here to produce endogenous GHB. However, when GHB is consumed for recreational or health promotion purposes, its concentration in the body is much higher than normal, which changes the enzyme kinetics so that these pathways operate to metabolise GHB rather than producing it.
History
Alexander Zaytsev worked on this chemical family and published work on it in 1874. The first extended research into GHB and its use in humans was conducted in the early 1960s by Henri Laborit to use in studying the neurotransmitter GABA. It was studied in a range of uses including obstetric surgery and during childbirth and as an anxiolytic; there were anecdotal reports of it having antidepressant and aphrodisiac effects as well. It was also studied as an intravenous anesthetic agent and was marketed for that purpose starting in 1964 in Europe but it was not widely adopted as it caused seizures; as of 2006 that use was still authorized in France and Italy but not widely used. It was also studied to treat alcohol addiction; while the evidence for this use is weak; however, sodium oxybate is marketed for this use in Italy.
GHB and sodium oxybate were also studied for use in narcolepsy from the 1960s onwards.
In May 1990 GHB was introduced as a dietary supplement and was marketed to body builders, for help with weight control and as a sleep aid, and as a "replacement" for l-tryptophan, which was removed from the market in November 1989 when batches contaminated with trace impurities were found to cause eosinophilia–myalgia syndrome, although eosinophilia–myalgia syndrome is also tied to tryptophan overload. In 2001 tryptophan supplement sales were allowed to resume, and in 2005 the FDA ban on tryptophan supplement importation was lifted. By November 1989 57 cases of illness caused by the GHB supplements had been reported to the Centers for Disease Control and Prevention, with people having taken up to three teaspoons of GHB; there were no deaths but nine people needed care in an intensive care unit. The FDA issued a warning in November 1990 that sale of GHB was illegal. GHB continued to be manufactured and sold illegally and it and analogs were adopted as a club drug and came to be used as a date rape drug, and the DEA made seizures and the FDA reissued warnings several times throughout the 1990s.
At the same time, research on the use of GHB in the form of sodium oxybate had formalized, as a company called Orphan Medical had filed an investigational new drug application and was running clinical trials with the intention of gaining regulatory approval for use to treat narcolepsy.
A popular children's toy, Bindeez (also known as Aqua Dots, in the United States), produced by Melbourne company Moose, was banned in Australia in early November 2007 when it was discovered that 1,4-butanediol (1,4-B), which is metabolized into GHB, had been substituted for the non-toxic plasticiser 1,5-pentanediol in the bead manufacturing process. Three young children were hospitalized as a result of ingesting a large number of the beads, and the toy was recalled.
Legal status
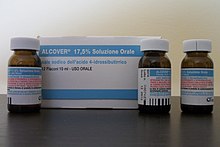
In the United States, GHB was placed on Schedule I of the Controlled Substances Act in March 2000. However, used in sodium oxybate under an IND or NDA from the US FDA, it is considered a Schedule III substance but with Schedule I trafficking penalties, one of several drugs that are listed in multiple schedules.
On 20 March 2001, the UN Commission on Narcotic Drugs placed GHB in Schedule IV of the 1971 Convention on Psychotropic Substances.
In the UK GHB was made a class C drug in June 2003. In October 2013 the ACMD recommended upgrading it from schedule IV to schedule II in line with UN recommendations. Their report concluded that the minimal use of Xyrem in the UK meant that prescribers would be minimally inconvenienced by the rescheduling. This advice was followed and GHB was moved to schedule 2 on 7 January 2015. In April 2022 GHB was changed from class C to class B.
In Hong Kong, GHB is regulated under Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance. It can only be used legally by health professionals and for university research purposes. The substance can be given by pharmacists under a prescription. Anyone who supplies the substance without prescription can be fined HK$10,000. The penalty for trafficking or manufacturing the substance is a HK$150,000 fine and life imprisonment. Possession of the substance for consumption without license from the Department of Health is illegal with a HK$100,000 fine or five years of jail time.
In Canada, GHB has been a Schedule I controlled substance since 6 November 2012 (the same schedule that contains heroin and cocaine). Prior to that date, it was a Schedule III controlled substance (the same schedule that contains amphetamines and LSD).
In New Zealand and Australia, GHB, 1,4-B, and GBL are all Class B illegal drugs, along with any possible esters, ethers, and aldehydes. GABA itself is also listed as an illegal drug in these jurisdictions, which seems unusual given its failure to cross the blood–brain barrier, but there was a perception among legislators that all known analogues should be covered as far as this was possible. Attempts to circumvent the illegal status of GHB have led to the sale of derivatives such as 4-methyl-GHB (γ-hydroxyvaleric acid, GHV) and its prodrug form γ-valerolactone (GVL), but these are also covered under the law by virtue of their being "substantially similar" to GHB or GBL, so importation, sale, possession and use of these compounds is also considered to be illegal.
In Chile, GHB is a controlled drug under the law Ley de substancias psicotrópicas y estupefacientes (psychotropic substances and narcotics).
In Norway and in Switzerland, GHB is considered a narcotic and is only available by prescription under the trade name Xyrem (Union Chimique Belge S.A.).
Sodium oxybate is also used therapeutically in Italy under the brand name Alcover for treatment of alcohol withdrawal and dependence.
See also
- Beta-Hydroxybutyric acid
- γ-Hydroxyvaleric acid (GHV)
- γ-Valerolactone (GVL)
- β-Hydroxy β-methylbutyric acid (HMB)
References
- "Pingers, pingas, pingaz: how drug slang affects the way we use and understand drugs". The Conversation. 8 January 2020. Archived from the original on 15 January 2021. Retrieved 13 May 2023.
- "GHB/GBL "G"". Archived from the original on 24 May 2024. Retrieved 24 May 2024.
- Tay, E., Lo, W. K. W., & Murnion, B. (2022). Current Insights on the Impact of Gamma-Hydroxybutyrate (GHB) Abuse. Substance Abuse and Rehabilitation, 13, 13–23. doi:10.2147/SAR.S315720
- "Therapeutic Goods (Poisons Standard—June 2024) Instrument 2024". 30 May 2024.
- Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- "What is GHB?" (PDF). Dea.gov. Archived (PDF) from the original on 17 October 2020. Retrieved 6 March 2022.
- ^ "2000 – Addition of Gamma-Hydroxybutyric Acid to Schedule I". US Department of Justice via the Federal Register. 13 March 2000. Archived from the original on 1 May 2021. Retrieved 16 April 2018.
- Riviello RJ (2010). Manual of forensic emergency medicine : a guide for clinicians. Sudbury, MA: Jones and Bartlett Publishers. p. 42. ISBN 978-0763744625.
- ^ Busardò FP, Jones AW (January 2015). "GHB pharmacology and toxicology: acute intoxication, concentrations in blood and urine in forensic cases and treatment of the withdrawal syndrome". Current Neuropharmacology. 13 (1): 47–70. doi:10.2174/1570159X13666141210215423. PMC 4462042. PMID 26074743.
- "Sodium Oxybate: MedlinePlus Drug Information". Nlm.nih.gov. 28 July 2010. Archived from the original on 11 April 2010. Retrieved 1 August 2010.
- ^ Benzer TI, Cameron S (8 January 2007). VanDeVoort JT, Benitez JG (eds.). "Toxicity, Gamma-Hydroxybutyrate". eMedicine. Archived from the original on 28 November 2021. Retrieved 16 January 2007.
- ^ US Drug Enforcement Administration. "GHB, GBL and 1,4BD as Date Rape Drugs". Archived from the original on 10 May 2012. Retrieved 10 May 2012.
- Weil A, Winifred R (1993). "Depressants". From Chocolate to Morphine (2nd ed.). Boston/New York: Houghton Mifflin Company. p. 77. ISBN 978-0-395-66079-9.
- Mayer G (May 2012). "The use of sodium oxybate to treat narcolepsy". Expert Review of Neurotherapeutics. 12 (5): 519–29. doi:10.1586/ern.12.42. PMID 22550980. S2CID 43706704.
- Caputo F, Mirijello A, Cibin M, Mosti A, Ceccanti M, Domenicali M, et al. (April 2015). "Novel strategies to treat alcohol dependence with sodium oxybate according to clinical practice". European Review for Medical and Pharmacological Sciences. 19 (7): 1315–20. PMID 25912595.
- Keating GM (January 2014). "Sodium oxybate: a review of its use in alcohol withdrawal syndrome and in the maintenance of abstinence in alcohol dependence". Clinical Drug Investigation. 34 (1): 63–80. doi:10.1007/s40261-013-0158-x. PMID 24307430. S2CID 2056246.
- Leone MA, Vigna-Taglianti F, Avanzi G, Brambilla R, Faggiano F, et al. (Cochrane Drugs and Alcohol Group) (February 2010). "Gamma-hydroxybutyrate (GHB) for treatment of alcohol withdrawal and prevention of relapses". The Cochrane Database of Systematic Reviews (2): CD006266. doi:10.1002/14651858.CD006266.pub2. PMID 20166080.
There is insufficient randomised evidence to be confident of a difference between GHB and placebo, or to determine reliably if GHB is more or less effective than other drugs for the treatment of alcohol withdrawl [sic?] or the prevention of relapses.
- Leone MA, Vigna-Taglianti F, Avanzi G, Brambilla R, Faggiano F (2010). "cochranelibrary.com". The Cochrane Database of Systematic Reviews (2): CD006266. doi:10.1002/14651858.CD006266.pub2. PMID 20166080. Archived from the original on 5 November 2022. Retrieved 23 January 2023.
- Calandre EP, Rico-Villademoros F, Slim M (June 2015). "An update on pharmacotherapy for the treatment of fibromyalgia". Expert Opinion on Pharmacotherapy. 16 (9): 1347–68. doi:10.1517/14656566.2015.1047343. PMID 26001183. S2CID 24246355.
- Staud R (August 2011). "Sodium oxybate for the treatment of fibromyalgia". Expert Opinion on Pharmacotherapy. 12 (11): 1789–98. doi:10.1517/14656566.2011.589836. PMID 21679091. S2CID 33026097.
- "FDA Approval Letter for Xyrem; Indication: Cataplexy associated with narcolepsy; 17 July 2002" (PDF). Archived (PDF) from the original on 17 October 2012. Retrieved 6 March 2022.
- "FDA Approval Letter for Xyrem; Indication: EDS (Excessive Daytime Sleepiness) associated with narcolepsy; 18 November 2005" (PDF). Archived (PDF) from the original on 17 October 2012. Retrieved 6 March 2022.
- ^ Scrima L, Hartman PG, Johnson FH, Thomas EE, Hiller FC (December 1990). "The effects of gamma-hydroxybutyrate on the sleep of narcolepsy patients: a double-blind study". Sleep. 13 (6): 479–90. doi:10.1093/sleep/13.6.479. PMID 2281247.
- Scrima L, Johnson FH, Hiller FC (1991). "Long-Term Effect of Gamma-Hydroxybutyrate on Sleep in Narcolepsy Patients". Sleep Research. 20: 330.
- Van Cauter E, Plat L, Scharf MB, Leproult R, Cespedes S, L'Hermite-Balériaux M, et al. (August 1997). "Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men". The Journal of Clinical Investigation. 100 (3): 745–53. doi:10.1172/JCI119587. PMC 508244. PMID 9239423.
- Scrima L, Shander D (January 1991). "Letter to Editor on article: Re: Narcolepsy Review (Aldrich MS: 8-9-91)". The New England Journal of Medicine. 324 (4): 270–72. doi:10.1056/nejm199101243240416. PMID 1985252.
- "FDA Approved Labeling Text: Xyrem® (sodium oxybate) oral solution" (PDF). U.S. Food and Drug Administration. 18 November 2005. Archived (PDF) from the original on 24 January 2022. Retrieved 23 April 2021.
- Guiraud J, Addolorato G, Aubin HJ, Batel P, de Bejczy A, Caputo F, et al. (July 2021). "Treating alcohol dependence with an abuse and misuse deterrent formulation of sodium oxybate: Results of a randomised, double-blind, placebo-controlled study". European Neuropsychopharmacology. 52: 18–30. doi:10.1016/j.euroneuro.2021.06.003. hdl:11392/2483205. PMID 34237655.
- ^ Schep LJ, Knudsen K, Slaughter RJ, Vale JA, Mégarbane B (July 2012). "The clinical toxicology of γ-hydroxybutyrate, γ-butyrolactone and 1,4-butanediol". Clinical Toxicology. 50 (6): 458–70. doi:10.3109/15563650.2012.702218. PMID 22746383. S2CID 19697449.
- Sellman JD, Robinson GM, Beasley R (January 2009). "Should ethanol be scheduled as a drug of high risk to public health?". Journal of Psychopharmacology. 23 (1): 94–100. doi:10.1177/0269881108091596. PMID 18583435.
- ^ Galloway GP, Frederick-Osborne SL, Seymour R, Contini SE, Smith DE (April 2000). "Abuse and therapeutic potential of gamma-hydroxybutyric acid". Alcohol. 20 (3): 263–69. doi:10.1016/S0741-8329(99)00090-7. PMID 10869868.
- Thai D, Dyer JE, Benowitz NL, Haller CA (October 2006). "Gamma-hydroxybutyrate and ethanol effects and interactions in humans". Journal of Clinical Psychopharmacology. 26 (5): 524–29. doi:10.1097/01.jcp.0000237944.57893.28. PMC 2766839. PMID 16974199.
- "The Vaults Of Erowid" Archived 22 November 2015 at the Wayback Machine. Erowid.org (18 March 2009). Retrieved on 27 September 2012.
- US 4393236, Klosa, Joseph, "Production of nonhygroscopic salts of 4-hydroxybutyric acid", issued 12 July 1983
- "1,4-Butanediol". PubChem. U.S National Library of Medicine. Archived from the original on 1 February 2021. Retrieved 15 January 2021.
- Kapoor P, Deshmukh R, Kukreja I (October 2013). "GHB acid: A rage or reprive". Journal of Advanced Pharmaceutical Technology & Research. 4 (4): 173–178. doi:10.4103/2231-4040.121410. PMC 3853692. PMID 24350046.
- ^ Jones C (June 2001). "Suspicious death related to gamma-hydroxybutyrate (GHB) toxicity". Journal of Clinical Forensic Medicine. 8 (2): 74–76. doi:10.1054/jcfm.2001.0473. PMID 15274975.
- Kam PC, Yoong FF (December 1998). "Gamma-hydroxybutyric acid: an emerging recreational drug". Anaesthesia. 53 (12): 1195–98. doi:10.1046/j.1365-2044.1998.00603.x. PMID 10193223.
In the UK, GHB has been available in the night clubs around London since 1994...
- Carter LP, Pardi D, Gorsline J, Griffiths RR (September 2009). "Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse". Drug and Alcohol Dependence. 104 (1–2): 1–10. doi:10.1016/j.drugalcdep.2009.04.012. PMC 2713368. PMID 19493637.
- Klein M, Kramer F (February 2004). "Rave drugs: pharmacological considerations". AANA Journal. 72 (1): 61–67. PMID 15098519.
- "Warning on Risk of 'Party Drug' Chemicals". The New York Times. The Associated Press. 12 May 1999. Archived from the original on 6 April 2023. Retrieved 16 April 2018.
- ^ Németh Z, Kun B, Demetrovics Z (September 2010). "The involvement of gamma-hydroxybutyrate in reported sexual assaults: a systematic review". Journal of Psychopharmacology. 24 (9): 1281–7. doi:10.1177/0269881110363315. PMID 20488831. S2CID 25496192.
- ElSohly MA, Salamone SJ (1999). "Prevalence of drugs used in cases of alleged sexual assault". Journal of Analytical Toxicology. 23 (3): 141–6. doi:10.1093/jat/23.3.141. PMID 10369321.
- "No evidence to suggest widespread date rape drug use'". 16 November 2006. Archived from the original on 8 January 2009. Retrieved 8 April 2014.
- "Date-rape drugs 'not widespread'". BBC News. 16 November 2006. Archived from the original on 20 May 2007. Retrieved 8 April 2014.
- Alcohol and other popular Date Rape Drugs. udel.edu
- "Labs making date-rape drug raided". The Independent. 10 July 2008. Archived from the original on 25 September 2015.
- Smith JL, Greene S, McCutcheon D, Weber C, Kotkis E, Soderstrom J, et al. (March 2024). "A multicentre case series of analytically confirmed gamma-hydroxybutyrate intoxications in Western Australian emergency departments: Pre-hospital circumstances, co-detections and clinical outcomes". Drug and Alcohol Review. 43 (4): 984–996. doi:10.1111/dar.13830. PMID 38426636.
- Martin JH (16 January 2009). "Remembering Samantha Reid: 10th anniversary of teen's GHB death". thenewsherald.com. Archived from the original on 4 March 2016. Retrieved 27 September 2012.
- "Stephen Port: Serial killer guilty of murdering four men". BBC News. 23 November 2016.
- Kintz P, Cirimele V, Jamey C, Ludes B (January 2003). "Testing for GHB in hair by GC/MS/MS after a single exposure. Application to document sexual assault" (PDF). Journal of Forensic Sciences. 48 (1): 195–200. doi:10.1520/JFS2002209. PMID 12570228. Archived from the original (PDF) on 24 December 2012.
- "Drink Speaks the Truth: Forensic Investigation of Drug Facilitated Sexual Assaults". 20 June 2013. Archived from the original on 14 March 2016. Retrieved 24 July 2014.
- Lott S, Piper T, Mehling LM, Spottke A, Maas A, Thevis M, et al. (2015). "Measurement of exogenous gamma-hydroxybutyric acid (GHB) in urine using isotope ratio mass spectrometry (IRMS)" (PDF). Toxichem Krimtech. 82: 264. Archived (PDF) from the original on 4 March 2016.
- Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. S2CID 5667719.
- Poldrugo F, Addolorato G (1999). "The role of gamma-hydroxybutyric acid in the treatment of alcoholism: from animal to clinical studies". Alcohol and Alcoholism. 34 (1): 15–24. doi:10.1093/alcalc/34.1.15. PMID 10075397.
- Zvosec et al. Proceedings of the American Academy of Forensic Science in Seattle, 2006. Web.archive.org (3 December 2007). Retrieved on 24 December 2011.
- Zvosec DL, Smith SW, Porrata T, Strobl AQ, Dyer JE (March 2011). "Case series of 226 γ-hydroxybutyrate-associated deaths: lethal toxicity and trauma". The American Journal of Emergency Medicine. 29 (3): 319–32. doi:10.1016/j.ajem.2009.11.008. PMID 20825811.
- Zvosec DL, Smith SW, Hall BJ (April 2009). "Three deaths associated with use of Xyrem". Sleep Medicine. 10 (4): 490–93. doi:10.1016/j.sleep.2009.01.005. PMID 19269893.
- Feldman NT (April 2009). "Xyrem safety: the debate continues". Sleep Medicine. 10 (4): 405–06. doi:10.1016/j.sleep.2009.02.002. PMID 19332385.
- Zvosec DL, Smith SW (January 2010). "Response to Editorial: "Xyrem safety: The debate continues"". Sleep Medicine. 11 (1): 108, author reply 108–09. doi:10.1016/j.sleep.2009.08.004. PMID 19959395.
- ^ Sircar R, Basak A (December 2004). "Adolescent gamma-hydroxybutyric acid exposure decreases cortical N-methyl-D-aspartate receptor and impairs spatial learning". Pharmacology, Biochemistry, and Behavior. 79 (4): 701–08. doi:10.1016/j.pbb.2004.09.022. PMID 15582677. S2CID 31736568.
- García FB, Pedraza C, Arias JL, Navarro JF (August 2006). "". Psicothema (in Spanish). 18 (3): 519–24. PMID 17296081.
- Sircar R, Basak A, Sircar D (October 2008). "Gamma-hydroxybutyric acid-induced cognitive deficits in the female adolescent rat". Annals of the New York Academy of Sciences. 1139 (1): 386–89. Bibcode:2008NYASA1139..386S. doi:10.1196/annals.1432.044. PMID 18991885. S2CID 1823886.
- Sgaravatti AM, Sgarbi MB, Testa CG, Durigon K, Pederzolli CD, Prestes CC, et al. (February 2007). "Gamma-hydroxybutyric acid induces oxidative stress in cerebral cortex of young rats". Neurochemistry International. 50 (3): 564–70. doi:10.1016/j.neuint.2006.11.007. PMID 17197055. S2CID 43049617.
- Sgaravatti AM, Magnusson AS, Oliveira AS, Mescka CP, Zanin F, Sgarbi MB, et al. (June 2009). "Effects of 1,4-butanediol administration on oxidative stress in rat brain: study of the neurotoxicity of gamma-hydroxybutyric acid in vivo". Metabolic Brain Disease. 24 (2): 271–82. doi:10.1007/s11011-009-9136-7. PMID 19296210. S2CID 13460935.
- Department of Health and Human Services, SAMHSA Office of Applied Studies 2005 National Survey on Drug Use and Health (ages 12 years and up); American Heart Association; Johns Hopkins University study, Principles of Addiction Medicine; Psychology Today; National Gambling Impact Commission Study; National Council on Problem Gambling; Illinois Institute for Addiction Recovery; Society for Advancement of Sexual Health; All Psych Journal
- Addiction and the Brain. Time
- Colombo G, Agabio R, Balaklievskaia N, Diaz G, Lobina C, Reali R, et al. (October 1995). "Oral self-administration of gamma-hydroxybutyric acid in the rat". European Journal of Pharmacology. 285 (1): 103–07. doi:10.1016/0014-2999(95)00493-5. PMID 8846805.
- "Is GHB toxic? Addictive? Dangerous?". lycaeum.org. Archived from the original on 27 January 2011.
- Galloway GP, Frederick SL, Staggers FE, Gonzales M, Stalcup SA, Smith DE (January 1997). "Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence". Addiction. 92 (1): 89–96. doi:10.1111/j.1360-0443.1997.tb03640.x. PMID 9060200.
- "GHB: An Important Pharmacologic and Clinical Update". Ualberta.ca. Archived from the original on 4 March 2016. Retrieved 1 August 2010.
- Santos C, Olmedo RE (March 2017). "Sedative-Hypnotic Drug Withdrawal Syndrome: Recognition And Treatment". Emergency Medicine Practice. 19 (3): 1–20. PMID 28186869.
- LeTourneau JL, Hagg DS, Smith SM (2008). "Baclofen and gamma-hydroxybutyrate withdrawal". Neurocritical Care. 8 (3): 430–33. doi:10.1007/s12028-008-9062-2. PMC 2630388. PMID 18266111.
- Carter LP, Koek W, France CP (January 2009). "Behavioral analyses of GHB: receptor mechanisms". Pharmacology & Therapeutics. 121 (1): 100–14. doi:10.1016/j.pharmthera.2008.10.003. PMC 2631377. PMID 19010351.
- van Noorden MS, van Dongen LC, Zitman FG, Vergouwen TA (2009). "Gamma-hydroxybutyrate withdrawal syndrome: dangerous but not well-known". General Hospital Psychiatry. 31 (4): 394–96. doi:10.1016/j.genhosppsych.2008.11.001. PMID 19555805.
- Allen L, Alsalim W (April 2006). "Best evidence topic report. Gammahydroxybutyrate overdose and physostigmine". Emergency Medicine Journal. 23 (4): 300–01. doi:10.1136/emj.2006.035139. PMC 2579509. PMID 16549578.
- Michael H, Harrison M (January 2005). "Best evidence topic report: endotracheal intubation in gamma-hydroxybutyric acid intoxication and overdose". Emergency Medicine Journal. 22 (1): 43. doi:10.1136/emj.2004.021154. PMC 1726538. PMID 15611542.
- Morse BL, Vijay N, Morris ME (August 2012). "γ-Hydroxybutyrate (GHB)-induced respiratory depression: combined receptor-transporter inhibition therapy for treatment in GHB overdose". Molecular Pharmacology. 82 (2): 226–235. doi:10.1124/mol.112.078154. PMC 3400846. PMID 22561075.
- Zvosec DL, Smith SW, Porrata T, Strobl AQ, Dyer JE (March 2011). "Case series of 226 γ-hydroxybutyrate-associated deaths: lethal toxicity and trauma". The American Journal of Emergency Medicine. 29 (3): 319–332. doi:10.1016/j.ajem.2009.11.008. PMID 20825811.
- Allen MJ, Sabir S, Sharma S (2022). "GABA Receptor". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 30252380. Archived from the original on 1 December 2022. Retrieved 21 October 2022.
- Carai MA, Colombo G, Gessa GL (June 2005). "Resuscitative effect of a gamma-aminobutyric acid B receptor antagonist on gamma-hydroxybutyric acid mortality in mice". Annals of Emergency Medicine. 45 (6): 614–19. doi:10.1016/j.annemergmed.2004.12.013. PMID 15940094.
- Couper FJ, Thatcher JE, Logan BK (September 2004). "Suspected GHB overdoses in the emergency department". Journal of Analytical Toxicology. 28 (6): 481–84. doi:10.1093/jat/28.6.481. PMID 15516299.
- Marinetti LJ, Isenschmid DS, Hepler BR, Kanluen S (2005). "Analysis of GHB and 4-methyl-GHB in postmortem matrices after long-term storage". Journal of Analytical Toxicology. 29 (1): 41–47. doi:10.1093/jat/29.1.41. PMID 15808012.
- R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 680–84.
- "New spit test for 'date rape' drug developed in the UK". BBC News. August 2016. Archived from the original on 8 November 2020. Retrieved 9 January 2016.
- Kemmel V, Taleb O, Perard A, Andriamampandry C, Siffert JC, Mark J, et al. (October 1998). "Neurochemical and electrophysiological evidence for the existence of a functional gamma-hydroxybutyrate system in NCB-20 neurons". Neuroscience. 86 (3): 989–1000. doi:10.1016/S0306-4522(98)00085-2. PMID 9692734. S2CID 21001043.
- Löscher W (2002). "Valproic Acid: Mechanism of Action". In Levy RH, Mattson RH, Meldrum BS, Perucca E (eds.). Antiepileptic drugs (5th ed.). Philadelphia: Lippincott Williams & Wilkins. p. 774. ISBN 978-0-7817-2321-3. Archived from the original on 18 August 2023. Retrieved 16 August 2021.
- National Organization for Rare Disorders. Succinic Semialdehyde Dehydrogenase Deficiency Archived 6 April 2023 at the Wayback Machine. Retrieved 6 March 2010.
- Andriamampandry C, Taleb O, Viry S, Muller C, Humbert JP, Gobaille S, et al. (September 2003). "Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator gamma-hydroxybutyrate (GHB)". FASEB Journal. 17 (12): 1691–93. doi:10.1096/fj.02-0846fje. PMID 12958178. S2CID 489179.
- ^ Castelli MP, Ferraro L, Mocci I, Carta F, Carai MA, Antonelli T, et al. (November 2003). "Selective gamma-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of gamma-hydroxybutyric acid". Journal of Neurochemistry. 87 (3): 722–732. doi:10.1046/j.1471-4159.2003.02037.x. PMID 14535954. S2CID 82175813.
- Maitre M, Ratomponirina C, Gobaille S, Hodé Y, Hechler V (April 1994). "Displacement of gamma-hydroxybutyrate binding by benzamide neuroleptics and prochlorperazine but not by other antipsychotics". European Journal of Pharmacology. 256 (2): 211–14. doi:10.1016/0014-2999(94)90248-8. PMID 7914168.
- Gobaille S, Hechler V, Andriamampandry C, Kemmel V, Maitre M (July 1999). "gamma-Hydroxybutyrate modulates synthesis and extracellular concentration of gamma-aminobutyric acid in discrete rat brain regions in vivo". The Journal of Pharmacology and Experimental Therapeutics. 290 (1): 303–09. PMID 10381791.
- Ottani A, Saltini S, Bartiromo M, Zaffe D, Renzo Botticelli A, Ferrari A, et al. (October 2003). "Effect of gamma-hydroxybutyrate in two rat models of focal cerebral damage". Brain Research. 986 (1–2): 181–90. doi:10.1016/S0006-8993(03)03252-9. PMID 12965243. S2CID 54374774.
- Elliott S, Burgess V (July 2005). "The presence of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) in alcoholic and non-alcoholic beverages". Forensic Science International. 151 (2–3): 289–92. doi:10.1016/j.forsciint.2005.02.014. PMID 15939164.
- Wu Y, Ali S, Ahmadian G, Liu CC, Wang YT, Gibson KM, et al. (December 2004). "Gamma-hydroxybutyric acid (GHB) and gamma-aminobutyric acidB receptor (GABABR) binding sites are distinctive from one another: molecular evidence". Neuropharmacology. 47 (8): 1146–56. doi:10.1016/j.neuropharm.2004.08.019. PMID 15567424. S2CID 54233206.
- Cash CD, Gobaille S, Kemmel V, Andriamampandry C, Maitre M (December 1999). "Gamma-hydroxybutyrate receptor function studied by the modulation of nitric oxide synthase activity in rat frontal cortex punches". Biochemical Pharmacology. 58 (11): 1815–19. doi:10.1016/S0006-2952(99)00265-8. PMID 10571257.
- ^ Maitre M, Humbert JP, Kemmel V, Aunis D, Andriamampandry C (March 2005). "[A mechanism for gamma-hydroxybutyrate (GHB) as a drug and a substance of abuse]". Médecine/Sciences (in French). 21 (3): 284–89. doi:10.1051/medsci/2005213284. PMID 15745703.
- Waszkielewicz A, Bojarski J (2004). "Gamma-hydrobutyric acid (GHB) and its chemical modifications: a review of the GHBergic system" (PDF). Polish Journal of Pharmacology. 56 (1): 43–49. PMID 15047976. Archived (PDF) from the original on 6 August 2021. Retrieved 3 June 2008.
- McGregor IS, Callaghan PD, Hunt GE (May 2008). "From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use?". British Journal of Pharmacology. 154 (2): 358–68. doi:10.1038/bjp.2008.132. PMC 2442436. PMID 18475254.
- Kuriyama K, Sze PY (January 1971). "Blood-brain barrier to H3-gamma-aminobutyric acid in normal and amino oxyacetic acid-treated animals". Neuropharmacology. 10 (1): 103–08. doi:10.1016/0028-3908(71)90013-X. PMID 5569303.
- ^ Gobaille S, Schleef C, Hechler V, Viry S, Aunis D, Maitre M (March 2002). "Gamma-hydroxybutyrate increases tryptophan availability and potentiates serotonin turnover in rat brain". Life Sciences. 70 (18): 2101–2112. doi:10.1016/s0024-3205(01)01526-0. PMID 12002803.
- Hedner T, Lundborg P (1983). "Effect of gammahydroxybutyric acid on serotonin synthesis, concentration and metabolism in the developing rat brain". Journal of Neural Transmission. 57 (1–2): 39–48. doi:10.1007/BF01250046. PMID 6194255. S2CID 9471705.
- Pardi D, Black J (2006). "gamma-Hydroxybutyrate/sodium oxybate: neurobiology, and impact on sleep and wakefulness". CNS Drugs. 20 (12): 993–1018. doi:10.2165/00023210-200620120-00004. PMID 17140279. S2CID 72211254.
- Dimitrijevic N, Dzitoyeva S, Satta R, Imbesi M, Yildiz S, Manev H (September 2005). "Drosophila GABA(B) receptors are involved in behavioral effects of gamma-hydroxybutyric acid (GHB)". European Journal of Pharmacology. 519 (3): 246–252. doi:10.1016/j.ejphar.2005.07.016. PMID 16129424.
- Pistis M, Muntoni AL, Pillolla G, Perra S, Cignarella G, Melis M, et al. (2005). "Gamma-hydroxybutyric acid (GHB) and the mesoaccumbens reward circuit: evidence for GABA(B) receptor-mediated effects". Neuroscience. 131 (2): 465–474. doi:10.1016/j.neuroscience.2004.11.021. PMID 15708487. S2CID 54342374.
- ^ Kamal RM, van Noorden MS, Franzek E, Dijkstra BA, Loonen AJ, De Jong CA (2016). "The Neurobiological Mechanisms of Gamma-Hydroxybutyrate Dependence and Withdrawal and Their Clinical Relevance: A Review". Neuropsychobiology. 73 (2): 65–80. doi:10.1159/000443173. hdl:2066/158441. PMID 27003176. S2CID 33389634.
- Banerjee PK, Snead OC (June 1995). "Presynaptic gamma-hydroxybutyric acid (GHB) and gamma-aminobutyric acidB (GABAB) receptor-mediated release of GABA and glutamate (GLU) in rat thalamic ventrobasal nucleus (VB): a possible mechanism for the generation of absence-like seizures induced by GHB". The Journal of Pharmacology and Experimental Therapeutics. 273 (3): 1534–1543. PMID 7791129.
- Hechler V, Gobaille S, Bourguignon JJ, Maitre M (March 1991). "Extracellular events induced by gamma-hydroxybutyrate in striatum: a microdialysis study". Journal of Neurochemistry. 56 (3): 938–944. doi:10.1111/j.1471-4159.1991.tb02012.x. PMID 1847191. S2CID 86392963.
- Maitre M, Hechler V, Vayer P, Gobaille S, Cash CD, Schmitt M, et al. (November 1990). "A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties". The Journal of Pharmacology and Experimental Therapeutics. 255 (2): 657–663. PMID 2173754.
- Míguez I, Aldegunde M, Duran R, Veira JA (June 1988). "Effect of low doses of gamma-hydroxybutyric acid on serotonin, noradrenaline, and dopamine concentrations in rat brain areas". Neurochemical Research. 13 (6): 531–533. doi:10.1007/BF00973292. PMID 2457177. S2CID 27073926.
- Smolders I, De Klippel N, Sarre S, Ebinger G, Michotte Y (September 1995). "Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat". European Journal of Pharmacology. 284 (1–2): 83–91. doi:10.1016/0014-2999(95)00369-V. PMID 8549640.
- Mamelak M (1989). "Gammahydroxybutyrate: an endogenous regulator of energy metabolism". Neuroscience and Biobehavioral Reviews. 13 (4): 187–198. doi:10.1016/S0149-7634(89)80053-3. PMID 2691926. S2CID 20217078.
- Oliveto A, Gentry WB, Pruzinsky R, Gonsai K, Kosten TR, Martell B, et al. (July 2010). "Behavioral effects of gamma-hydroxybutyrate in humans". Behavioural Pharmacology. 21 (4): 332–342. doi:10.1097/FBP.0b013e32833b3397. PMC 2911496. PMID 20526195.
- Wu H, Zink N, Carter LP, Mehta AK, Hernandez RJ, Ticku MK, et al. (May 2003). "A tertiary alcohol analog of gamma-hydroxybutyric acid as a specific gamma-hydroxybutyric acid receptor ligand". The Journal of Pharmacology and Experimental Therapeutics. 305 (2): 675–79. doi:10.1124/jpet.102.046797. PMID 12606613. S2CID 86191608.
- Felmlee MA, Morse BL, Morris ME (January 2021). "γ-Hydroxybutyric Acid: Pharmacokinetics, Pharmacodynamics, and Toxicology". The AAPS Journal. 23 (1): 22. doi:10.1208/s12248-020-00543-z. PMC 8098080. PMID 33417072.
- Taxon ES, Halbers LP, Parsons SM (May 2020). "Kinetics aspects of Gamma-hydroxybutyrate dehydrogenase". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1868 (5): 140376. doi:10.1016/j.bbapap.2020.140376. PMID 31981617.
- Kamal RM, van Noorden MS, Franzek E, Dijkstra BA, Loonen AJ, De Jong CA (March 2016). "The Neurobiological Mechanisms of Gamma-Hydroxybutyrate Dependence and Withdrawal and Their Clinical Relevance: A Review". Neuropsychobiology. 73 (2): 65–80. doi:10.1159/000443173. hdl:2066/158441. PMID 27003176.
- Lewis DE (2012). "Section 4.4.3 Aleksandr Mikhailovich Zaitsev". Early Russian organic chemists and their legacy. Springer. p. 79. ISBN 978-3642282195.
- Saytzeff A (1874). "Über die Reduction des Succinylchlorids". Liebigs Annalen der Chemie (in German). 171 (2): 258–90. doi:10.1002/jlac.18741710216. Archived from the original on 4 March 2021. Retrieved 30 June 2019.
- ^ "Critical review of gamma-hydroxybutyric acid (GHB)" (PDF). 2012. Archived (PDF) from the original on 10 September 2014.
- Laborit H, Jouany JM, Gerard J, Fabiani F (October 1960). "". Agressologie (in French). 1: 397–406. PMID 13758011.
- "Alcover: Riassunto delle Caratteristiche del Prodotto". Agenzia Italiana del Farmaco. 31 March 2017. Archived from the original on 6 August 2021. Retrieved 16 April 2018. Index page Archived 4 March 2021 at the Wayback Machine
- Ito J, Hosaki Y, Torigoe Y, Sakimoto K (January 1992). "Identification of substances formed by decomposition of peak E substance in tryptophan". Food and Chemical Toxicology. 30 (1): 71–81. doi:10.1016/0278-6915(92)90139-C. PMID 1544609.
- Smith MJ, Garrett RH (November 2005). "A heretofore undisclosed crux of eosinophilia-myalgia syndrome: compromised histamine degradation". Inflammation Research. 54 (11): 435–50. doi:10.1007/s00011-005-1380-7. PMID 16307217. S2CID 7785345.
- Kapalka GM (2010). Nutritional and herbal therapies for children and adolescents : a handbook for mental health clinicians. Elsevier/AP. ISBN 978-0-12-374927-7.
- ^ Centers for Disease Control (CDC) (November 1990). "Multistate outbreak of poisonings associated with illicit use of gamma hydroxy butyrate". MMWR. Morbidity and Mortality Weekly Report. 39 (47): 861–63. PMID 2122223. Archived from the original on 6 June 2021. Retrieved 16 April 2018.
- Dyer JE (July 1991). "gamma-Hydroxybutyrate: a health-food product producing coma and seizurelike activity". The American Journal of Emergency Medicine. 9 (4): 321–24. doi:10.1016/0735-6757(91)90050-t. PMID 2054002.
- Institute of Medicine, National Research Council (US) Committee on the Framework for Evaluating the Safety of Dietary Supplements (2002). "Appendix D: Table of Food and Drug Administration Actions on Dietary Supplements". Proposed Framework for Evaluating the Safety of Dietary Supplements: For Comment. National Academies Press (US). Archived from the original on 29 August 2021. Retrieved 16 April 2018.
- "GHB: A Club Drug To Watch" (PDF). Substance Abuse Treatment Advisory. 2 (1). November 2002. Archived from the original (PDF) on 1 August 2017. Retrieved 16 April 2018.
- Mason PE, Kerns WP (July 2002). "Gamma hydroxybutyric acid (GHB) intoxication". Academic Emergency Medicine. 9 (7): 730–79. doi:10.1197/aemj.9.7.730. PMID 12093716. S2CID 13325306.
- "Transcript: FDA Peripheral and Central Nervous System Drugs Advisory Committee Meeting". FDA. 6 June 2001. Archived from the original on 16 May 2017. Retrieved 16 April 2018.
- Perry M, Pomfret J, Crabb RP (7 November 2007). "Australia bans China-made toy on toxic drug risk". Reuters. Archived from the original on 5 April 2023. Retrieved 2 July 2017.
- "William J. Clinton: Statement on Signing the Hillory J. Farias and Samantha Reid Date-Rape Drug Prohibition Act of 2000". 18 February 2000. Archived from the original on 13 September 2018. Retrieved 16 April 2018.
- "Gamma Hydroxybutyrate (GHB)". 2002. Archived from the original on 31 December 2005. Retrieved 20 November 2016.
- Iversen L (3 October 2013). "ACMD advice on the scheduling of GHB" (PDF). UK Home Office. Archived from the original on 13 August 2014. Retrieved 23 October 2013.
- "Circular 001/2015: A Change to the Misuse of Drugs Act 1971: control of AH-7921, LSD–related compounds, tryptamines, and rescheduling of GHB". UK Home Office. 2015. Archived from the original on 6 April 2023. Retrieved 8 May 2017.
- "The Misuse of Drugs (Amendment No. 3) (England, Wales and Scotland) Regulations 2014". UK Home Office. 11 December 2014. Archived from the original on 5 August 2023. Retrieved 8 May 2017.
- "The Misuse of Drugs Act 1971 (Amendment) Order 2022". www.legislation.gov.uk. Archived from the original on 26 July 2023. Retrieved 15 June 2023.
- "Controlled Drugs and Substances Act, SC 1996, c 19". Canadian Legal Information Institute. Archived from the original on 17 June 2019. Retrieved 25 November 2018.
- "FOR 30 June 1978 nr 08: Forskrift om narkotika m.v. (Narkotikalisten)" (in Norwegian). Archived from the original on 16 October 2014. Retrieved 17 August 2008.
- Haller C, Thai D, Jacob P, Dyer JE (2006). "GHB urine concentrations after single-dose administration in humans". Journal of Analytical Toxicology. 30 (6): 360–64. doi:10.1093/jat/30.6.360. PMC 2257868. PMID 16872565.
External links
- Gamma-hydroxybutyrate MS Spectrum
- EMCDDA Report on the risk assessment of GHB in the framework of the joint action on new synthetic drugs
- Erowid GHB Vault (also contains information about addiction and dangers)
- InfoFacts – Rohypnol and GHB (National Institute on Drug Abuse)
| Neurotransmitters | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino acid-derived |
| ||||||||||||||||||||||
| Lipid-derived |
| ||||||||||||||||||||||
| Nucleobase-derived |
| ||||||||||||||||||||||
| Vitamin-derived | |||||||||||||||||||||||
| Miscellaneous |
| ||||||||||||||||||||||
| Hypnotics/sedatives (N05C) | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GABAA |
| ||||||||||||||||||||||||
| GABAB | |||||||||||||||||||||||||
| H1 |
| ||||||||||||||||||||||||
| α2-Adrenergic | |||||||||||||||||||||||||
| 5-HT2A |
| ||||||||||||||||||||||||
| Melatonin | |||||||||||||||||||||||||
| Orexin | |||||||||||||||||||||||||
| α2δ VDCC | |||||||||||||||||||||||||
| Others | |||||||||||||||||||||||||
| Drugs which induce euphoria | |
|---|---|
| |
| See also: Recreational drug use |
| GHB receptor modulators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor (ligands) |
| ||||||||||
| Transporter (blockers) |
| ||||||||||
| Enzyme (inhibitors) |
| ||||||||||
| GABA receptor modulators | |||||
|---|---|---|---|---|---|
| Ionotropic |
| ||||
| Metabotropic |
| ||||