This is an old revision of this page, as edited by Beetstra (talk | contribs) at 21:26, 6 August 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 21:26, 6 August 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)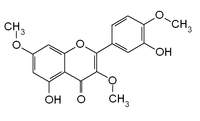
| |
| Names | |
|---|---|
| IUPAC name 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,7-dimethoxychromen-4-one | |
| Other names
3,7,4'-Tri-O-methylquercetin 3,7,4'-trimethylquercetin 5,3'-dihydroxy-3,7,4'-trimethoxyflavone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C18H16O7 |
| Molar mass | 344.31 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ayanin is a O-methylated flavonol, a type of flavonoid. It is the 3,7,4'-tri-O-methylation of quercetin.
It can be found in Croton schiedeanus. It can also be synthetized.
Biosynthetis
The enzyme 3,7-dimethylquercetin 4'-O-methyltransferase uses S-adenosyl methionine and 5,3',4'-trihydroxy-3,7-dimethoxyflavone (rhamnazin) to produce S-adenosylhomocysteine and 5,3'-dihydroxy-3,7,4'-trimethoxyflavone (ayanin).