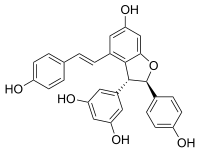
 (−)-trans-ε-Viniferin | |
| Names | |
|---|---|
| Preferred IUPAC name 5-{(2R,3R)-6-Hydroxy-2-(4-hydroxyphenyl)-4--2,3-dihydro-1-benzofuran-3-yl}benzene-1,3-diol | |
| Other names
Viniferin epsilon-Viniferin (−)-epsilon-Viniferin (−)-(E)-epsilon-viniferin trans-ε-viniferin (−)-Trans-epsilon-viniferin Iso--viniferin cis-ε-viniferin Cis-epsilon-viniferin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C28H22O6 |
| Molar mass | 454.478 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
ε-Viniferin is a naturally occurring phenol, belonging to the stilbenoids family. It is a resveratrol dimer.
It is found in Vitis vinifera grapevines, in wines, in the Oriental medicinal plant Vitis coignetiae and in the stem bark of Dryobalanops aromatica.
Cis-epsilon-viniferin can be found in Paeonia lactiflora.
It shows a human cytochrome P450 enzymes inhibition activity.
Glycosides
Diptoindonesin A is a C-glucoside of ε-viniferin.
See also
References
- Yáñez, M.; Fraiz, N.; Cano, E.; Orallo, F. (2006). "(−)-Trans-ε-viniferin, a polyphenol present in wines, is an inhibitor of noradrenaline and 5-hydroxytryptamine uptake and of monoamine oxidase activity". European Journal of Pharmacology. 542 (1–3): 54–60. doi:10.1016/j.ejphar.2006.06.005. PMID 16828740.
- Cornacchione, S.; Sadick, N. S.; Neveu, M.; Talbourdet, S.; Lazou, K.; Viron, C.; Renimel, I.; De Quéral, D.; Kurfurst, R.; Schnebert, S.; Heusèle, C.; André, P.; Perrier, E. (2007). "In vivo skin antioxidant effect of a new combination based on a specific Vitis vinifera shoot extract and a biotechnological extract". Journal of Drugs in Dermatology. 6 (6 Suppl): s8–13. PMID 17691204.
- ^ Kim, H. J.; Chang, E. J.; Cho, S. H.; Chung, S. K.; Park, H. D.; Choi, S. W. (2002). "Antioxidative Activity of Resveratrol and Its Derivatives Isolated from Seeds of Paeonia lactiflora". Bioscience, Biotechnology, and Biochemistry. 66 (9): 1990–3. doi:10.1271/bbb.66.1990. PMID 12400706. S2CID 24367582.
- Privat, C.; Telo, J. O. P.; Bernardes-Genisson, V.; Vieira, A.; Souchard, J. P.; Nepveu, F. O. (2002). "Antioxidant Properties oftrans-ε-Viniferin As Compared to Stilbene Derivatives in Aqueous and Nonaqueous Media". Journal of Agricultural and Food Chemistry. 50 (5): 1213–1217. doi:10.1021/jf010676t. PMID 11853506.
- Langcake, P.; Pryce, R. J. (1977). "A new class of phytoalexins from grapevines". Experientia. 33 (2): 151–152. doi:10.1007/BF02124034. PMID 844529. S2CID 34370048.
- Vitrac, X.; Bornet, A. L.; Vanderlinde, R.; Valls, J.; Richard, T.; Delaunay, J. C.; Mérillon, J. M.; Teissédre, P. L. (2005). "Determination of Stilbenes (δ-viniferin, trans-astringin, trans-piceid, cis- and trans-resveratrol, ε-viniferin) in Brazilian Wines". Journal of Agricultural and Food Chemistry. 53 (14): 5664–5669. doi:10.1021/jf050122g. PMID 15998130.
- Wibowo, A.; Ahmat, N.; Hamzah, A. S.; Sufian, A. S.; Ismail, N. H.; Ahmad, R.; Jaafar, F. M.; Takayama, H. (2011). "Malaysianol A, a new trimer resveratrol oligomer from the stem bark of Dryobalanops aromatica". Fitoterapia. 82 (4): 676–681. doi:10.1016/j.fitote.2011.02.006. PMID 21338657.
- Piver, B.; Berthou, F. O.; Dreano, Y.; Lucas, D. L. (2003). "Differential inhibition of human cytochrome P450 enzymes by ε-viniferin, the dimer of resveratrol: Comparison with resveratrol and polyphenols from alcoholized beverages". Life Sciences. 73 (9): 1199–1213. doi:10.1016/S0024-3205(03)00420-X. PMID 12818727.
External links
 Media related to Epsilon-Viniferin at Wikimedia Commons
Media related to Epsilon-Viniferin at Wikimedia Commons- e-viniferin on phenol-explorer.eu
| Oligostilbenoids and their glycosides | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimers |
| ||||||||||||
| Trimers | |||||||||||||
| Tetramers: |
| ||||||||||||
| Higher polymers (five units or more) |
| ||||||||||||
| Oligomeric forms of resveratrol |
| ||||||||||||
| Glycosides or conjugates |
| ||||||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |