
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name (2E)-3-Phenylprop-2-enoic acid | |
| Systematic IUPAC name Cinnamic acid | |
| Other names
trans-Cinnamic acid Phenylacrylic acid Cinnamylic acid 3-Phenylacrylic acid (E)-Cinnamic acid Benzenepropenoic acid Isocinnamic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1905952 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.908 |
| EC Number |
|
| Gmelin Reference | 3731 |
| IUPHAR/BPS | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H8O2 |
| Molar mass | 148.161 g·mol |
| Appearance | White monoclinic crystals |
| Odor | Honey-like |
| Density | 1.2475 g/cm |
| Melting point | 133 °C (271 °F; 406 K) |
| Boiling point | 300 °C (572 °F; 573 K) |
| Solubility in water | 500 mg/L |
| Acidity (pKa) | 4.44 |
| Magnetic susceptibility (χ) | −7.836×10 cm/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | > 100 °C (212 °F; 373 K) |
| Related compounds | |
| Related compounds | Benzoic acid, Phenylacetic acid, Phenylpropanoic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Cinnamic acid is an organic compound with the formula C6H5-CH=CH-COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.
Occurrence and production
Biosynthesis
Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine.
Natural occurrence
It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter. Cinnamic acid has a honey-like odor; and its more volatile ethyl ester, ethyl cinnamate, is a flavor component in the essential oil of cinnamon, in which related cinnamaldehyde is the major constituent. It is also found in wood from many diverse tree species.
Synthesis
Cinnamic acid was first synthesized by the base-catalysed condensation of acetyl chloride and benzaldehyde, followed by hydrolysis of the acid chloride product. In 1890, Rainer Ludwig Claisen described the synthesis of ethyl cinnamate via the reaction of ethyl acetate with benzaldehyde in the presence of sodium as base. Another way of preparing cinnamic acid is by the Knoevenagel condensation reaction. The reactants for this are benzaldehyde and malonic acid in the presence of a weak base, followed by acid-catalyzed decarboxylation. It can also be prepared by oxidation of cinnamaldehyde, condensation of benzal chloride and sodium acetate (followed by acid hydrolysis), and the Perkin reaction. The oldest commercially used route to cinnamic acid involves the Perkin reaction, which is given in the following scheme
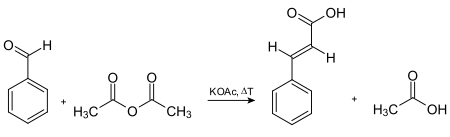
Synthesis of cinnamic acid via the Perkin reaction.
Metabolism
Cinnamic acid, obtained from autoxidation of cinnamaldehyde, is metabolized into sodium benzoate in the liver.
Uses
Cinnamic acid is used in flavorings, synthetic indigo, and certain pharmaceuticals. A major use is as a precursor to produce methyl cinnamate, ethyl cinnamate, and benzyl cinnamate for the perfume industry. Cinnamic acid is a precursor to the sweetener aspartame via enzyme-catalysed amination with phenylalanine. Cinnamic acid can dimerize in non-polar solvents resulting in different linear free energy relationships.
References
- "Cinnamic Acid" . Encyclopædia Britannica. Vol. 6 (11th ed.). 1911. p. 376.
- ^ "Cinnamic acid". flavornet.org.
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Budavari, Susan, ed. (1996). The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (12th ed.). Merck. ISBN 0911910123.
- ^ Garbe, Dorothea (2012). "Cinnamic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a07_099. ISBN 978-3527306732.
- Vogt, T. (2010). "Phenylpropanoid Biosynthesis". Molecular Plant. 3 (1): 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
- Oldach, Laurel (February 22, 2023). "Forensic researchers use mass spectrometry to identify smuggled wood". Chemical and Engineering News. American Chemical Society.
- Claisen, L. (1890). "Zur Darstellung der Zimmtsäure und ihrer Homologen" [On the preparation of cinnamic acid and its homologues]. Berichte der Deutschen Chemischen Gesellschaft. 23: 976–978. doi:10.1002/cber.189002301156.
- Tieze, L. (1988). Reactions and Synthesis in the Organic Chemistry Laboratory. Mill Vall, CA. p. 1988.
{{cite book}}: CS1 maint: location missing publisher (link) - F. K. Thayer (1925). "m-Nitrocinnamic Acid". Organic Syntheses. 5: 83. doi:10.15227/orgsyn.005.0083.
- Jana A, Modi KK, Roy A, Anderson JA, van Breemen RB, Pahan K (June 2013). "Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders". Journal of Neuroimmune Pharmacology. 8 (3): 739–55. doi:10.1007/s11481-013-9447-7. PMC 3663914. PMID 23475543.
- Bradley, J.-C.; Abraham, M. H.; Acree, W. E.; Lang, A.; Beck, S. N.; Bulger, D. A.; Clark, E. A.; Condron, L. N.; Costa, S. T.; Curtin, E. M.; Kurtu, S. B.; Mangir, M. I.; McBride, M. J. (2015). "Determination of Abraham model solute descriptors for the monomeric and dimeric forms of trans-cinnamic acid using measured solubilities from the Open Notebook Science Challenge". Chemistry Central Journal. 9: 11. doi:10.1186/s13065-015-0080-9. PMC 4369286. PMID 25798191.
| Types of hydroxycinnamic acids | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglycones |
| ||||||||||||||||
| Esters |
| ||||||||||||||||
| Oligomeric forms |
| ||||||||||||||||
| Conjugates with coenzyme A (CoA) | |||||||||||||||||