| Revision as of 06:48, 16 June 2015 view sourceOpabinia regalis (talk | contribs)Autopatrolled, Administrators16,306 editsm Reverted edits by 49.15.21.89 (talk) to last version by ClueBot NG← Previous edit | Latest revision as of 18:00, 4 December 2024 view source Kupirijo (talk | contribs)Extended confirmed users9,124 edits →Clastosomes: (κλαστός) (σῶμα) | ||
| (603 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|Eukaryotic membrane-bounded organelle containing DNA}} | |||
| {{pp-move-indef|small=yes}} | |||
| {{pp-vandalism|expiry=indef|small=yes}} | |||
| ] cells stained for the cell nucleus ] with the ] ] dye. The central and rightmost cell are in ], thus their entire nuclei are labeled. On the left, a cell is going through ] and its DNA has condensed.]] | |||
| {{Use dmy dates|date=August 2018}} | |||
| ] stained for ] with the blue ] ]. The central and rightmost cells are in ], thus their entire nuclei are labeled. On the left, a cell is going through ] and its DNA has condensed.]] | |||
| {{Organelle diagram}} | {{Organelle diagram}} | ||
| The '''cell nucleus''' ({{etymology|la|{{wikt-lang|la|nucleus}} or {{wikt-lang|la|nuculeus}}|kernel, seed}}; {{plural form}}: '''nuclei''') is a ] found in ] ]. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian ]s, have ], and a few others including ]s have ]. The main structures making up the nucleus are the ], a double membrane that encloses the entire organelle and isolates its contents from the cellular ]; and the ], a network within the nucleus that adds mechanical support. | |||
| The cell nucleus contains nearly all of the cell's ]. ] is often organized into multiple ]s – long strands of ] dotted with various ]s, such as ]s, that protect and organize the DNA. The ]s within these chromosomes are ] in such a way to promote cell function. The nucleus maintains the integrity of genes and controls the activities of the cell by regulating ]. | |||
| Because the nuclear |
Because the nuclear envelope is impermeable to ], ]s are required to regulate ] of molecules across the envelope. The pores cross both nuclear membranes, providing a ] through which larger molecules must be ] by carrier proteins while allowing free movement of small molecules and ]s. Movement of large molecules such as proteins and ] through the pores is required for both gene expression and the maintenance of chromosomes. Although the interior of the nucleus does not contain any membrane-bound subcompartments, a number of ] exist, made up of unique proteins, RNA molecules, and particular parts of the chromosomes. The best-known of these is the ], involved in the assembly of ]s. | ||
| == |
==Chromosomes== | ||
| {{Main|Chromosome}} | |||
| ], 1719]] | |||
| {{Further|Nuclear organization}} | |||
| ]'' salivary gland cell published by ] in 1882. The nucleus contains ]s. | |||
| ]] | |||
| The nucleus was the first organelle to be discovered. What is most likely the oldest preserved drawing dates back to the early microscopist ] (1632–1723). He observed a "Lumen", the nucleus, in the ]s of ].<ref>Leeuwenhoek, A. van: Opera Omnia, seu Arcana Naturae ope exactissimorum Microscopiorum detecta, experimentis variis comprobata, Epistolis ad varios illustres viros. J. Arnold et Delphis, A. Beman, Lugdinum Batavorum 1719–1730. Cited after: Dieter Gerlach, Geschichte der Mikroskopie. Verlag Harry Deutsch, Frankfurt am Main, Germany, 2009. ISBN 978-3-8171-1781-9.</ref> Unlike mammalian red blood cells, those of other vertebrates still possess nuclei. | |||
| The nucleus was also described by ] in 1804<ref name="Harris">{{cite book | last =Harris | first =H | title =The Birth of the Cell | edition = | year =1999 | publisher =Yale University Press | location =New Haven | isbn =0-300-07384-4 }}</ref> and in more detail in 1831 by Scottish ] ] in a talk at the ]. Brown was studying ]s under microscope when he observed an opaque area, which he called the areola or nucleus, in the cells of the flower's outer layer.<ref name="Robert Brown">{{cite journal | last = Brown | first = Robert | title = On the Organs and Mode of Fecundation of Orchidex and Asclepiadea | journal = Miscellaneous Botanical Works I | volume = | pages = 511–514 | year = 1866}}</ref> | |||
| He did not suggest a potential function. In 1838, ] proposed that the nucleus plays a role in generating cells, thus he introduced the name "Cytoblast" (cell builder). He believed that he had observed new cells assembling around "cytoblasts". ] was a strong opponent of this view, having already described cells multiplying by division and believing that many cells would have no nuclei. The idea that cells can be generated de novo, by the "cytoblast" or otherwise, contradicted work by ] (1852) and ] (1855) who decisively propagated the new paradigm that cells are generated solely by cells ("Omnis cellula e cellula"). The function of the nucleus remained unclear.<ref name="Cremer">{{cite book | last =Cremer| first =Thomas | title =Von der Zellenlehre zur Chromosomentheorie | edition = | year =1985 | publisher =Springer Verlag | location =Berlin, Heidelberg, New York, Tokyo | isbn = 3-540-13987-7}} Online Version </ref> | |||
| ] nucleus in which ] is stained blue. The distinct chromosome territories of chromosome 2 (red) and chromosome 9 (green) are stained with ].]] | |||
| Between 1877 and 1878, ] published several studies on the ] of ] eggs, showing that the nucleus of the ] enters the ] and fuses with its nucleus. This was the first time it was suggested that an individual develops from a (single) nucleated cell. This was in contradiction to ]'s theory that the complete ] of a species would be repeated during embryonic development, including generation of the first nucleated cell from a "Monerula", a structureless mass of primordial mucus ("Urschleim"). Therefore, the necessity of the sperm nucleus for fertilization was discussed for quite some time. However, Hertwig confirmed his observation in other animal groups, e.g., ] and ]. ] produced the same results for plants (1884). This paved the way to assign the nucleus an important role in heredity. In 1873, ] postulated the equivalence of the maternal and paternal germ ''cells'' for heredity. The function of the nucleus as carrier of genetic information became clear only later, after ] was discovered and the ] were rediscovered at the beginning of the 20th century; the chromosome theory of heredity was therefore developed.<ref name ="Cremer"/> | |||
| The cell nucleus contains the majority of the cell's genetic material in the form of multiple linear DNA molecules organized into structures called ]s. Each human cell contains roughly two meters of DNA.<ref name = "Lodish" />{{rp|405}} During most of the ] these are organized in a DNA-protein complex known as ], and during cell division the chromatin can be seen to form the well-defined chromosomes familiar from a ]. A small fraction of the cell's genes are located instead in the ].<ref name = "Lodish" />{{rp|438}} | |||
| ==Structures== | |||
| The nucleus is the largest cellular ] in animal cells.<ref name="Lodish">{{cite book | last = Lodish | first = H |author2=Berk A|author3=Matsudaira P|author4=Kaiser CA|author5=Krieger M|author6=Scott MP|author7=Zipursky SL|author8=Darnell J. | title = Molecular Cell Biology | publisher = WH Freeman | edition = 5th | year = 2004 | location = New York | isbn = 0-7167-2672-6}}</ref> | |||
| There are two types of chromatin. ] is the less compact DNA form, and contains genes that are frequently ] by the cell.<ref name="Ehrenhofer">{{cite journal | vauthors = Ehrenhofer-Murray AE | title = Chromatin dynamics at DNA replication, transcription and repair | journal = European Journal of Biochemistry | volume = 271 | issue = 12 | pages = 2335–49 | date = June 2004 | pmid = 15182349 | doi = 10.1111/j.1432-1033.2004.04162.x | department = Review | doi-access = free }}</ref> The other type, ], is the more compact form, and contains DNA that is infrequently transcribed. This structure is further categorized into ], consisting of genes that are organized as heterochromatin only in certain cell types or at certain stages of development, and ] that consists of chromosome structural components such as ]s and ]s.<ref name="Grigoryev">{{cite journal | vauthors = Grigoryev SA, Bulynko YA, Popova EY | title = The end adjusts the means: heterochromatin remodelling during terminal cell differentiation | journal = Chromosome Research | volume = 14 | issue = 1 | pages = 53–69 | year = 2006 | pmid = 16506096 | doi = 10.1007/s10577-005-1021-6 | s2cid = 6040822 | department = Review }}</ref> During interphase the chromatin organizes itself into discrete individual patches,<ref name="Schardin">{{cite journal | vauthors = Schardin M, Cremer T, Hager HD, Lang M | title = Specific staining of human chromosomes in Chinese hamster x man hybrid cell lines demonstrates interphase chromosome territories | journal = Human Genetics | volume = 71 | issue = 4 | pages = 281–7 | date = December 1985 | pmid = 2416668 | doi = 10.1007/BF00388452 | url = https://epub.ub.uni-muenchen.de/9272/1/cremer_thomas_9272.pdf | s2cid = 9261461 | department = Primary }}</ref> called '']''.<ref name="Lamond">{{cite journal | vauthors = Lamond AI, Earnshaw WC | title = Structure and function in the nucleus | journal = Science | volume = 280 | issue = 5363 | pages = 547–53 | date = April 1998 | pmid = 9554838 | doi = 10.1126/science.280.5363.547 | url = http://azolla.fc.ul.pt/aulas/BiologiaCelular/docs/nucleo.pdf | citeseerx = 10.1.1.323.5543 | department = Review }}</ref> Active genes, which are generally found in the euchromatic region of the chromosome, tend to be located towards the chromosome's territory boundary.<ref name="Kurz">{{cite journal | vauthors = Kurz A, Lampel S, Nickolenko JE, Bradl J, Benner A, Zirbel RM, Cremer T, Lichter P | display-authors = 6 | title = Active and inactive genes localize preferentially in the periphery of chromosome territories | journal = The Journal of Cell Biology | volume = 135 | issue = 5 | pages = 1195–205 | date = December 1996 | pmid = 8947544 | pmc = 2121085 | doi = 10.1083/jcb.135.5.1195 | url = http://intl.jcb.org/cgi/content/abstract/135/5/1195 | url-status = dead | department = Primary | archive-url = https://web.archive.org/web/20070929104104/http://intl.jcb.org/cgi/content/abstract/135/5/1195 | archive-date = 29 September 2007 }}</ref> | |||
| In ]ian cells, the average diameter of the nucleus is approximately 6 ]s (µm), which occupies about 10% of the total cell volume.<ref name="MBoC">{{cite book | year = 2002 | title = Molecular Biology of the Cell, Chapter 4, pages 191–234 | editor = Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, Peter Walter | publisher = Garland Science | edition = 4th}}</ref> The viscous liquid within it is called ], and is similar in composition to the ] found outside the nucleus.<ref>{{cite journal |author=Clegg JS |title=Properties and metabolism of the aqueous cytoplasm and its boundaries |journal=Am. J. Physiol. |volume=246 |issue=2 Pt 2 |pages=R133–51 |date=February 1984 |pmid=6364846 |url=http://ajpregu.physiology.org/cgi/pmidlookup?view=reprint&pmid=6364846}}</ref> It appears as a dense, roughly spherical or irregular organelle. | |||
| Antibodies to certain types of chromatin organization, in particular, ]s, have been associated with a number of ]s, such as ].<ref name="Rothfield">{{cite journal | vauthors = Rothfield NF, Stollar BD | title = The relation of immunoglobulin class, pattern of anti-nuclear antibody, and complement-fixing antibodies to DNA in sera from patients with systemic lupus erythematosus | journal = The Journal of Clinical Investigation | volume = 46 | issue = 11 | pages = 1785–94 | date = November 1967 | pmid = 4168731 | pmc = 292929 | doi = 10.1172/JCI105669 | department = Primary }}</ref> These are known as ] (ANA) and have also been observed in concert with ] as part of general immune system dysfunction.<ref name="Barned">{{cite journal | vauthors = Barned S, Goodman AD, Mattson DH | title = Frequency of anti-nuclear antibodies in multiple sclerosis | journal = Neurology | volume = 45 | issue = 2 | pages = 384–5 | date = February 1995 | pmid = 7854544 | doi = 10.1212/WNL.45.2.384 | s2cid = 30482028 | department = Primary }}</ref> | |||
| ==Nuclear structures and landmarks== | |||
| {{Further|Nuclear equivalence}} | |||
| ]-studded ], ], ] (complexed as ]), and the ].]] | |||
| The nucleus contains nearly all of the cell's ], surrounded by a network of fibrous ] called the ], and is enveloped in a double membrane called the ]. The nuclear envelope separates the fluid inside the nucleus, called the ], from the rest of the cell. The size of the nucleus is correlated to the size of the cell, and this ] is reported across a range of cell types and species.<ref name="Kume">{{cite journal |vauthors=Kume K, Cantwell H, Neumann FR, Jones AW, Snijders AP, Nurse P |title=A systematic genomic screen implicates nucleocytoplasmic transport and membrane growth in nuclear size control |journal=PLOS Genet |volume=13 |issue=5 |pages=e1006767 |date=May 2017 |pmid=28545058 |pmc=5436639 |doi=10.1371/journal.pgen.1006767 |url= |doi-access=free }}</ref> In eukaryotes the nucleus in many cells typically occupies 10% of the cell volume.<ref name=Alberts2015/>{{rp|178}} The nucleus is the largest ] in animal cells.<ref name="Lodish_2016"/>{{rp|12}} In human cells, the diameter of the nucleus is approximately six ]s (μm).<ref name=Alberts2015/>{{rp|179}} | |||
| ===Nuclear envelope and pores=== | ===Nuclear envelope and pores=== | ||
| {{ |
{{Main|Nuclear envelope|Nuclear pore}} | ||
| ] on the surface of the ] (1). Other diagram labels show (2) the outer ring, (3) spokes, (4) basket, and (5) filaments.]] | |||
| {| align="right" valign="top" | |||
| | ]-studded ] of the nuclear envelope, the ] (complexed as ]), and the ]. Within the cell nucleus is a viscous liquid called ], similar to the cytoplasm found outside the nucleus.]] | |||
| | ] on the surface of the ] (1). Other diagram labels show (2) the outer ring, (3) spokes, (4) basket, and (5) filaments.]] | |||
| |} | |||
| The ] |
The ] consists of two ], an ] and an ], perforated by ]s.<ref name=Alberts2015>{{cite book|title=Molecular Biology of the Cell |edition=6 |vauthors=Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P |publisher=Garland Science |date=2015 |location=New York}}</ref>{{rp|649}} Together, these membranes serve to separate the cell's genetic material from the rest of the cell contents, and allow the nucleus to maintain an environment distinct from the rest of the cell. Despite their close apposition around much of the nucleus, the two membranes differ substantially in shape and contents. The inner membrane surrounds the nuclear content, providing its defining edge.<ref name="Lodish_2016"/>{{rp|14}} Embedded within the inner membrane, various proteins bind the intermediate filaments that give the nucleus its structure.<ref name=Alberts2015/>{{rp|649}} The outer membrane encloses the inner membrane, and is continuous with the adjacent ] membrane.<ref name=Alberts2015/>{{rp|649}} As part of the endoplasmic reticulum membrane, the outer nuclear membrane is studded with ]s that are actively translating proteins across membrane.<ref name=Alberts2015/>{{rp|649}} The space between the two membranes is called the perinuclear space, and is continuous with the endoplasmic reticulum ].<ref name=Alberts2015/>{{rp|649}} | ||
| ] |
In a mammalian nuclear envelope there are between 3000 and 4000 ]es (NPCs) perforating the envelope.<ref name=Alberts2015/>{{rp|650}} Each NPC contains an eightfold-symmetric ring-shaped structure at a position where the inner and outer membranes fuse.<ref name="Shulga">{{cite journal | vauthors = Shulga N, Mosammaparast N, Wozniak R, Goldfarb DS | title = Yeast nucleoporins involved in passive nuclear envelope permeability | journal = The Journal of Cell Biology | volume = 149 | issue = 5 | pages = 1027–38 | date = May 2000 | pmid = 10831607 | pmc = 2174828 | doi = 10.1083/jcb.149.5.1027 | department = Primary }}</ref> The number of NPCs can vary considerably across cell types; small ]s only have about a few hundred, with large ]s having around 20,000.<ref name=Alberts2015/>{{rp|650}} The NPC provides selective transport of molecules between the ] and the ].<ref name="Alberts2019">{{cite book |last1=Alberts |first1=Bruce |title=Essential cell biology |date=2019 |location=New York |isbn=9780393680393 |page=242 |edition=Fifth}}</ref> The nuclear pore complex is composed of approximately thirty different proteins known as ]s.<ref name=Alberts2015/>{{rp|649}} The pores are about 60–80 million ] in ] and consist of around 50 (in ]) to several hundred proteins (in ]s).<ref name = "Lodish_2016">{{cite book | vauthors = Lodish HF, Berk A, Kaiser C, Krieger M, Bretscher A, Ploegh H, Amon A, Martin KC, Darnell JE | display-authors = 6 | title = Molecular Cell Biology | date = 2016 | publisher = W.H. Freeman | location = New York | isbn = 978-1-4641-8339-3 | edition = Eighth }}</ref>{{rp|622–4}} The pores are 100 nm in total diameter; however, the gap through which molecules freely diffuse is only about 9 nm wide, due to the presence of regulatory systems within the center of the pore. This size selectively allows the passage of small water-soluble molecules while preventing larger molecules, such as ]s and larger proteins, from inappropriately entering or exiting the nucleus. These large molecules must be actively transported into the nucleus instead. Attached to the ring is a structure called the ''nuclear basket'' that extends into the nucleoplasm, and a series of filamentous extensions that reach into the cytoplasm. Both structures serve to mediate binding to nuclear transport proteins.<ref name="Lodish">{{cite book | vauthors = Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky SL, Darnell J | title = Molecular Cell Biology | publisher = WH Freeman | edition = 5th | year = 2004 | location = New York | isbn = 978-0-7167-2672-2 | url-access = registration | url = https://archive.org/details/studentcompanion0000unse_r7k2 }}</ref>{{rp|509–10}} | ||
| Most proteins, ribosomal subunits, and some |
Most proteins, ribosomal subunits, and some RNAs are transported through the pore complexes in a process mediated by a family of transport factors known as ]s. Those karyopherins that mediate movement into the nucleus are also called importins, whereas those that mediate movement out of the nucleus are called exportins. Most karyopherins interact directly with their cargo, although some use ].<ref name="Pemberton">{{cite journal | vauthors = Pemberton LF, Paschal BM | title = Mechanisms of receptor-mediated nuclear import and nuclear export | journal = Traffic | volume = 6 | issue = 3 | pages = 187–98 | date = March 2005 | pmid = 15702987 | doi = 10.1111/j.1600-0854.2005.00270.x | s2cid = 172279 | department = Review | doi-access = free }}</ref> ]s such as ] and ], as well as other small lipid-soluble molecules involved in intercellular ], can diffuse through the cell membrane and into the cytoplasm, where they bind ] proteins that are trafficked into the nucleus. There they serve as ]s when bound to their ]; in the absence of a ligand, many such receptors function as ]s that repress gene expression.<ref name="Lodish"/>{{rp|488}} | ||
| ===Nuclear lamina=== | ===Nuclear lamina=== | ||
| {{ |
{{Main|Nuclear lamina}} | ||
| In animal cells, two networks of ] provide the nucleus with mechanical support: The ] forms an organized meshwork on the internal face of the envelope, while less organized support is provided on the cytosolic face of the envelope. Both systems provide structural support for the nuclear envelope and anchoring sites for chromosomes and nuclear pores.<ref name="MBoC" /> | In animal cells, two networks of ] provide the nucleus with mechanical support: The ] forms an organized meshwork on the internal face of the envelope, while less organized support is provided on the cytosolic face of the envelope. Both systems provide structural support for the nuclear envelope and anchoring sites for chromosomes and nuclear pores.<ref name="MBoC">{{cite book | year = 2002 | title = Molecular Biology of the Cell | chapter = Chapter 4: DNA and Chromosomes | pages = 191–234 | veditors = Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P | location = New York | publisher = Garland Science | edition = 4th | isbn = 978-0-8153-4072-0 }}</ref> | ||
| The nuclear lamina is composed mostly of ] proteins. Like all proteins, lamins are synthesized in the cytoplasm and later transported to the nucleus interior, where they are assembled before being incorporated into the existing network of nuclear lamina.<ref name="Sturrman">{{cite |
The nuclear lamina is composed mostly of ] proteins. Like all proteins, lamins are synthesized in the cytoplasm and later transported to the nucleus interior, where they are assembled before being incorporated into the existing network of nuclear lamina.<ref name="Sturrman">{{cite journal | vauthors = Stuurman N, Heins S, Aebi U | title = Nuclear lamins: their structure, assembly, and interactions | journal = Journal of Structural Biology | volume = 122 | issue = 1–2 | pages = 42–66 | year = 1998 | pmid = 9724605 | doi = 10.1006/jsbi.1998.3987 | department = Review }}</ref><ref name="Goldman">{{cite journal | vauthors = Goldman AE, Moir RD, Montag-Lowy M, Stewart M, Goldman RD | title = Pathway of incorporation of microinjected lamin A into the nuclear envelope | journal = The Journal of Cell Biology | volume = 119 | issue = 4 | pages = 725–35 | date = November 1992 | pmid = 1429833 | pmc = 2289687 | doi = 10.1083/jcb.119.4.725 | department = Primary }}</ref> Lamins found on the cytosolic face of the membrane, such as ] and ], bind to the cytoskeleton to provide structural support. Lamins are also found inside the nucleoplasm where they form another regular structure, known as the ''nucleoplasmic veil'',<ref name="RGoldman">{{cite journal | vauthors = Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP | title = Nuclear lamins: building blocks of nuclear architecture | journal = Genes & Development | volume = 16 | issue = 5 | pages = 533–47 | date = March 2002 | pmid = 11877373 | doi = 10.1101/gad.960502 | doi-access = free | department = Review }}</ref><ref name="Broers_2004">{{cite journal | vauthors = Broers JL, Ramaekers FC | title = Dynamics of nuclear lamina assembly and disassembly | journal = Symposia of the Society for Experimental Biology | issue = 56 | pages = 177–92 | date = 2004 | pmid = 15565881 | isbn = 9781134279838 | url = https://books.google.com/books?id=lpR5AgAAQBAJ&pg=PA189 | department = Review }}</ref> that is visible using ]. The actual function of the veil is not clear, although it is excluded from the nucleolus and is present during ].<ref name="Moir">{{cite journal | vauthors = Moir RD, Yoon M, Khuon S, Goldman RD | title = Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells | journal = The Journal of Cell Biology | volume = 151 | issue = 6 | pages = 1155–68 | date = December 2000 | pmid = 11121432 | pmc = 2190592 | doi = 10.1083/jcb.151.6.1155 | department = Primary }}</ref> Lamin structures that make up the veil, such as ], bind chromatin and disrupting their structure inhibits transcription of protein-coding genes.<ref name="Spann">{{cite journal | vauthors = Spann TP, Goldman AE, Wang C, Huang S, Goldman RD | title = Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription | journal = The Journal of Cell Biology | volume = 156 | issue = 4 | pages = 603–8 | date = February 2002 | pmid = 11854306 | pmc = 2174089 | doi = 10.1083/jcb.200112047 | department = Primary }}</ref> | ||
| Like the components of other |
Like the components of other intermediate filaments, the lamin ] contains an ] domain used by two monomers to coil around each other, forming a ] structure called a ]. Two of these dimer structures then join side by side, in an ] arrangement, to form a ] called a ''protofilament''. Eight of these protofilaments form a lateral arrangement that is twisted to form a ropelike ''filament''. These filaments can be assembled or disassembled in a dynamic manner, meaning that changes in the length of the filament depend on the competing rates of filament addition and removal.<ref name="MBoC" /> | ||
| Mutations in lamin genes leading to defects in filament assembly cause a group of rare genetic disorders known as '']''. The most notable laminopathy is the family of diseases known as ], which causes the appearance of premature ] in |
Mutations in lamin genes leading to defects in filament assembly cause a group of rare genetic disorders known as '']''. The most notable laminopathy is the family of diseases known as ], which causes the appearance of premature ] in those with the condition. The exact mechanism by which the associated ] changes give rise to the aged ] is not well understood.<ref name="Mounkes">{{cite journal | vauthors = Mounkes LC, Stewart CL | title = Aging and nuclear organization: lamins and progeria | journal = Current Opinion in Cell Biology | volume = 16 | issue = 3 | pages = 322–7 | date = June 2004 | pmid = 15145358 | doi = 10.1016/j.ceb.2004.03.009 | url = https://zenodo.org/record/1258830 | department = Review }}</ref> | ||
| === |
===Nucleolus=== | ||
| {{ |
{{Main|Nucleolus}} | ||
| {{Further|Nuclear bodies}} | |||
| ] of a cell nucleus, showing the darkly stained ]]] | |||
| The ] is the largest of the discrete densely stained, membraneless structures known as ] found in the nucleus. It forms around ]s of ], DNA coding for ] (rRNA). These regions are called ] (NOR). The main roles of the nucleolus are to synthesize rRNA and ]. The structural cohesion of the nucleolus depends on its activity, as ribosomal assembly in the nucleolus results in the transient association of nucleolar components, facilitating further ribosomal assembly, and hence further association. This model is supported by observations that inactivation of rDNA results in intermingling of nucleolar structures.<ref name="Hernandez-Verdun">{{cite journal | vauthors = Hernandez-Verdun D | title = Nucleolus: from structure to dynamics | journal = Histochemistry and Cell Biology | volume = 125 | issue = 1–2 | pages = 127–37 | date = January 2006 | pmid = 16328431 | doi = 10.1007/s00418-005-0046-4 | url = https://hal.archives-ouvertes.fr/hal-00015455 | s2cid = 20769260 | department = Review }}</ref> | |||
| ] nucleus in which ] is stained blue. The distinct chromosome territories of chromosome 2 (red) and chromosome 9 (green) are stained with ].]] | |||
| In the first step of ribosome assembly, a protein called ] transcribes rDNA, which forms a large pre-rRNA precursor. This is cleaved into two ] – ], and ], and a ] ].<ref name=Alberts2015/>{{rp|328}}<ref name="Lamond-Sleeman">{{cite journal | vauthors = Lamond AI, Sleeman JE | title = Nuclear substructure and dynamics | journal = Current Biology | volume = 13 | issue = 21 | pages = R825-8 | date = October 2003 | pmid = 14588256 | doi = 10.1016/j.cub.2003.10.012 | s2cid = 16865665 | department = Review | doi-access = free | bibcode = 2003CBio...13.R825L }}</ref> The transcription, post-transcriptional processing, and assembly of rRNA occurs in the nucleolus, aided by ] (snoRNA) molecules, some of which are derived from spliced ]s from ]s encoding genes related to ribosomal function. The assembled ribosomal subunits are the largest structures passed through the ]s.<ref name="Lodish" />{{rp|526}} | |||
| The cell nucleus contains the majority of the cell's genetic material in the form of multiple linear ] molecules organized into structures called ]s. Each human cell contains roughly two meters of DNA. During most of the ] these are organized in a DNA-protein complex known as ], and during cell division the chromatin can be seen to form the well-defined ]s familiar from a ]. A small fraction of the cell's genes are located instead in the ]. | |||
| When observed under the ], the nucleolus can be seen to consist of three distinguishable regions: the innermost ''fibrillar centers'' (FCs), surrounded by the ''dense fibrillar component'' (DFC) (that contains ] and ]), which in turn is bordered by the ''granular component'' (GC) (that contains the protein ]). Transcription of the rDNA occurs either in the FC or at the FC-DFC boundary, and, therefore, when rDNA transcription in the cell is increased, more FCs are detected. Most of the cleavage and modification of rRNAs occurs in the DFC, while the latter steps involving protein assembly onto the ribosomal subunits occur in the GC.<ref name=Lamond-Sleeman /> | |||
| There are two types of chromatin. ] is the less compact DNA form, and contains genes that are frequently ] by the cell.<ref name="Ehrenhofer">{{cite journal | author = Ehrenhofer-Murray A | title = Chromatin dynamics at DNA replication, transcription and repair | journal = Eur J Biochem | volume = 271 | issue = 12 | pages = 2335–2349 | year = 2004 | pmid = 15182349 | doi = 10.1111/j.1432-1033.2004.04162.x }}</ref> The other type, ], is the more compact form, and contains DNA that is infrequently transcribed. This structure is further categorized into ], consisting of genes that are organized as heterochromatin only in certain cell types or at certain stages of development, and ] that consists of chromosome structural components such as ]s and ]s.<ref name="Grigoryev">{{cite journal | author = Grigoryev S, Bulynko Y, Popova E | title = The end adjusts the means: heterochromatin remodelling during terminal cell differentiation | journal = Chromosome Res | volume = 14 | issue = 1 | pages = 53–69 | year = 2006 | pmid = 16506096 | doi = 10.1007/s10577-005-1021-6 }}</ref> During interphase the chromatin organizes itself into discrete individual patches,<ref name="Schardin">{{cite journal | last = Schardin | first = Margit | authorlink = | title = Specific staining of human chromosomes in Chinese hamster x man hybrid cell lines demonstrates interphase chromosome territories | journal = Human Genetics | volume = 71 | issue = 4 | pages = 281–287 | publisher = Springer Berlin / Heidelberg |date=December 1985 | url = http://www.springerlink.com/content/lv101t8w17306071/ | doi = 10.1007/BF00388452 | pmid = 2416668 | last2 = Cremer | first2 = T | last3 = Hager | first3 = HD | last4 = Lang | first4 = M}}</ref> called ''chromosome territories''.<ref name="Lamond">{{cite journal | last = Lamond | first = Angus I. |author2=William C. Earnshaw | title = Structure and Function in the Nucleus | journal = Science | volume = 280 | pages = 547–553 | date = 1998-04-24 | pmid =9554838 | doi = 10.1126/science.280.5363.547 | issue=5363}}</ref> Active genes, which are generally found in the euchromatic region of the chromosome, tend to be located towards the chromosome's territory boundary.<ref name="Kurz">{{cite journal | last = Kurz | first = A | title = Active and inactive genes localize preferentially in the periphery of chromosome territories | doi = 10.1083/jcb.135.5.1195 | journal = The Journal of Cell Biology | volume = 135 | issue = 5| pages = 1195–1205 | publisher = The Rockefeller University Press | year = 1996 | url = http://intl.jcb.org/cgi/content/abstract/135/5/1195 | pmid =8947544 | last2 = Lampel | first2 = S | last3 = Nickolenko | first3 = JE | last4 = Bradl | first4 = J | last5 = Benner | first5 = A | last6 = Zirbel | first6 = RM | last7 = Cremer | first7 = T | last8 = Lichter | first8 = P | pmc = 2121085 }}</ref> | |||
| ==={{anchor|Splicing speckles}} Splicing speckles=== | |||
| Antibodies to certain types of chromatin organization, in particular, ]s, have been associated with a number of ]s, such as ].<ref name="Rothfield">{{cite journal | author = NF Rothfield, BD Stollar | title = The Relation of Immunoglobulin Class, Pattern of Antinuclear Antibody, and Complement-Fixing Antibodies to DNA in Sera from Patients with Systemic Lupus Erythematosus | journal = J Clin Invest | year = 1967 | volume = 46 | issue = 11 | pages = 1785–1794 | pmid = 4168731 | doi = 10.1172/JCI105669 | pmc = 292929 }}</ref> These are known as ] (ANA) and have also been observed in concert with ] as part of general immune system dysfunction.<ref name="Barned">{{cite journal | author = S Barned, AD Goodman, DH Mattson | title = Frequency of anti-nuclear antibodies in multiple sclerosis | journal = Neurology | year = 1995 | volume = 45 | issue = 2 | pages = 384–385 | pmid = 7854544 | doi = 10.1212/WNL.45.2.384}}</ref> As in the case of ], the role played by the antibodies in inducing the symptoms of autoimmune diseases is not obvious. | |||
| Speckles are subnuclear structures that are enriched in pre-messenger RNA splicing factors and are located in the interchromatin regions of the nucleoplasm of mammalian cells.<ref> | |||
| ===Nucleolus=== | |||
| {{cite journal | vauthors = Spector DL, Lamond AI | title = Nuclear speckles | journal = Cold Spring Harbor Perspectives in Biology | volume = 3 | issue = 2 | pages = a000646 | date = Feb 2011 | pmid = 20926517 | pmc = 3039535 | doi = 10.1101/cshperspect.a000646 | department = Review }}</ref> | |||
| {{main|Nucleolus}} | |||
| At the fluorescence-microscope level they appear as irregular, punctate structures, which vary in size and shape, and when examined by electron microscopy they are seen as clusters of ]. Speckles are dynamic structures, and both their protein and RNA-protein components can cycle continuously between speckles and other nuclear locations, including active transcription sites. Speckles can work with ] as enhancers of gene activity to directly enhance the activity of certain genes. Moreover, speckle-associating and non-associating p53 gene targets are functionally distinct.<ref> | |||
| {{cite journal | vauthors = Alexander KA, Coté A, Nguyen SC, Zhang L, Berger SL | title = p53 mediates target gene association with nuclear speckles for amplified RNA expression | journal = Molecular Cell | volume = 81| issue = 8| pages = S1097-2765(21)00174-X | date = Mar 2021 | pmid = 33823140 | pmc = 8830378| doi = 10.1016/j.molcel.2021.03.006 | s2cid = 233172170 | department = Primary }}</ref> | |||
| Studies on the composition, structure and behaviour of speckles have provided a model for understanding the functional compartmentalization of the nucleus and the organization of the gene-expression machinery<ref name="ReferenceA">{{cite journal | vauthors = Lamond AI, Spector DL | title = Nuclear speckles: a model for nuclear organelles | journal = Nature Reviews. Molecular Cell Biology | volume = 4 | issue = 8 | pages = 605–12 | date = August 2003 | pmid = 12923522 | doi = 10.1038/nrm1172 | s2cid = 6439413 | department = Review }}</ref> splicing ]s<ref>{{cite journal | vauthors = Tripathi K, Parnaik VK | title = Differential dynamics of splicing factor SC35 during the cell cycle | journal = Journal of Biosciences | volume = 33 | issue = 3 | pages = 345–54 | date = September 2008 | pmid = 19005234 | doi = 10.1007/s12038-008-0054-3 | url = http://www.ias.ac.in/jbiosci/sep2008/345.pdf | url-status = live | s2cid = 6332495 | department = Primary | archive-url = https://web.archive.org/web/20111115235056/http://www.ias.ac.in/jbiosci/sep2008/345.pdf | archive-date = 15 November 2011 }}</ref><ref>{{cite journal | vauthors = Tripathi K, Parnaik VK | title = Differential dynamics of splicing factor SC35 during the cell cycle | journal = Journal of Biosciences | volume = 33 | issue = 3 | pages = 345–54 | date = September 2008 | pmid = 19005234 | doi = 10.1007/s12038-008-0054-3 | s2cid = 6332495 | department = Primary }}</ref> and other splicing proteins necessary for pre-mRNA processing.<ref name="ReferenceA"/> Because of a cell's changing requirements, the composition and location of these bodies changes according to mRNA transcription and regulation via ] of specific proteins.<ref name="Handwerger">{{cite journal | vauthors = Handwerger KE, Gall JG | title = Subnuclear organelles: new insights into form and function | journal = Trends in Cell Biology | volume = 16 | issue = 1 | pages = 19–26 | date = January 2006 | pmid = 16325406 | doi = 10.1016/j.tcb.2005.11.005 | department = Review }}</ref> The splicing speckles are also known as nuclear speckles (nuclear specks), splicing factor compartments (SF compartments), interchromatin granule clusters (IGCs), and ].<ref>{{cite web | title = Cellular component Nucleus speckle | publisher = UniProt: UniProtKB | url = https://www.uniprot.org/locations/SL-0186 | access-date = 30 August 2013}}</ref> | |||
| ] of a cell nucleus, showing the darkly stained ]]] | |||
| B snurposomes are found in the amphibian oocyte nuclei and in '']'' embryos. B snurposomes appear alone or attached to the Cajal bodies in the electron micrographs of the amphibian nuclei.<ref> | |||
| {{cite journal | vauthors = Gall JG, Bellini M, Wu Z, Murphy C | title = Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes | journal = Molecular Biology of the Cell | volume = 10 | issue = 12 | pages = 4385–402 | date = December 1999 | pmid = 10588665 | pmc = 25765 | doi = 10.1091/mbc.10.12.4385 | department = Primary }}</ref> While nuclear speckles were originally thought to be storage sites for the splicing factors,<ref name="Matera2007_NatureMolCellBio">{{cite journal | vauthors = Matera AG, Terns RM, Terns MP | title = Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs | journal = Nature Reviews. Molecular Cell Biology | volume = 8 | issue = 3 | pages = 209–20 | date = March 2007 | pmid = 17318225 | doi = 10.1038/nrm2124 | s2cid = 30268055 | department = Review }}</ref> a more recent study demonstrated that organizing genes and pre-mRNA substrates near speckles increases the kinetic efficiency of pre-mRNA splicing, ultimately boosting protein levels by modulation of splicing.<ref>{{cite journal | vauthors = Bhat P, Chow A, Emert B et al | title = Genome organization around nuclear speckles drives mRNA splicing efficiency. | journal = Nature | volume = 629 | issue = 5 | pages = 1165–1173 | date = May 2024 | pmid = 38720076 | pmc = 11164319 | doi = 10.1038/s41586-024-07429-6 }}</ref> | |||
| ===Cajal bodies and gems=== | |||
| ] | |||
| ] | |||
| A nucleus typically contains between one and ten compact structures called ] or coiled bodies (CB), whose diameter measures between 0.2 μm and 2.0 μm depending on the cell type and species.<ref name="Cioce" /> When seen under an electron microscope, they resemble balls of tangled thread<ref name="Pollard" /> and are dense foci of distribution for the protein ].<ref name="MateraFrey">{{cite journal | vauthors = Matera AG, Frey MR | title = Coiled bodies and gems: Janus or gemini? | journal = American Journal of Human Genetics | volume = 63 | issue = 2 | pages = 317–21 | date = August 1998 | pmid = 9683623 | pmc = 1377332 | doi = 10.1086/301992 | department = Review }}</ref> CBs are involved in a number of different roles relating to RNA processing, specifically ] (snoRNA) and ] (snRNA) maturation, and histone mRNA modification.<ref name="Cioce" /> | |||
| Similar to Cajal bodies are Gemini of Cajal bodies, or gems, whose name is derived from the ] in reference to their close "twin" relationship with CBs. Gems are similar in size and shape to CBs, and in fact are virtually indistinguishable under the microscope.<ref name="MateraFrey" /> Unlike CBs, gems do not contain ] (snRNPs), but do contain a protein called ] (SMN) whose function relates to snRNP biogenesis. Gems are believed to assist CBs in snRNP biogenesis,<ref name="Matera">{{cite journal | vauthors = Matera AG | title = Of coiled bodies, gems, and salmon | journal = Journal of Cellular Biochemistry | volume = 70 | issue = 2 | pages = 181–92 | date = August 1998 | pmid = 9671224 | doi = 10.1002/(sici)1097-4644(19980801)70:2<181::aid-jcb4>3.0.co;2-k | s2cid = 44941483 | department = Review }}</ref> though it has also been suggested from microscopy evidence that CBs and gems are different manifestations of the same structure.<ref name="MateraFrey" /> Later ultrastructural studies have shown gems to be twins of Cajal bodies with the difference being in the coilin component; Cajal bodies are SMN positive and coilin positive, and gems are SMN positive and coilin negative.<ref name="Navascues">{{cite journal | vauthors = Navascues J, Berciano MT, Tucker KE, Lafarga M, Matera AG | title = Targeting SMN to Cajal bodies and nuclear gems during neuritogenesis | journal = Chromosoma | volume = 112 | issue = 8 | pages = 398–409 | date = June 2004 | pmid = 15164213 | pmc = 1592132 | doi = 10.1007/s00412-004-0285-5 | department = Primary }}</ref> | |||
| The ] is a discrete densely stained structure found in the nucleus. It is not surrounded by a membrane, and is sometimes called a ''suborganelle''. It forms around ] repeats of rDNA, DNA coding for ] (rRNA). These regions are called ] (NOR). The main roles of the nucleolus are to synthesize rRNA and assemble ribosomes. The structural cohesion of the nucleolus depends on its activity, as ribosomal assembly in the nucleolus results in the transient association of nucleolar components, facilitating further ribosomal assembly, and hence further association. This model is supported by observations that inactivation of rDNA results in intermingling of nucleolar structures.<ref name="Hernandez-Verdun">{{cite journal | |||
| | last = Hernandez-Verdun | |||
| | first = Daniele | |||
| | title = Nucleolus: from structure to dynamics | |||
| | journal =Histochem. Cell. Biol | |||
| | issue = 1–2 | |||
| | pages = 127–137 | |||
| | year = 2006 | |||
| | doi = 10.1007/s00418-005-0046-4 | |||
| | volume = 125 | |||
| | pmid = 16328431 }}</ref> | |||
| ===Other nuclear bodies=== | |||
| In the first step of ribosome assembly, a protein called ] transcribes rDNA, which forms a large pre-rRNA precursor. This is cleaved into the subunits 5.8S, 18S, and 28S rRNA.<ref name="Lamond-Sleeman">{{cite journal | |||
| | last = Lamond | |||
| | first = Angus I. | |||
| |author2=Judith E. Sleeman | |||
| | title = Nuclear substructure and dynamics | |||
| | journal = current biology | |||
| | volume = 13 | |||
| | issue = 21 | |||
| | pages = R825–828 | |||
| | pmid = 14588256 | |||
| | accessdate = | doi = 10.1016/j.cub.2003.10.012 | |||
| | date=October 2003}}</ref> The transcription, post-transcriptional processing, and assembly of rRNA occurs in the nucleolus, aided by ] (snoRNA) molecules, some of which are derived from spliced ]s from ]s encoding genes related to ribosomal function. The assembled ribosomal subunits are the largest structures passed through the nuclear pores.<ref name="Lodish" /> | |||
| {{main|Nuclear bodies}} | |||
| When observed under the ], the nucleolus can be seen to consist of three distinguishable regions: the innermost ''fibrillar centers'' (FCs), surrounded by the ''dense fibrillar component'' (DFC), which in turn is bordered by the ''granular component'' (GC). Transcription of the rDNA occurs either in the FC or at the FC-DFC boundary, and, therefore, when rDNA transcription in the cell is increased, more FCs are detected. Most of the cleavage and modification of rRNAs occurs in the DFC, while the latter steps involving protein assembly onto the ribosomal subunits occur in the GC.<ref name=Lamond-Sleeman /> | |||
| ===Other subnuclear bodies=== | |||
| {| class="wikitable" style="float:right; font-size:100%; margin-left:15px;" | {| class="wikitable" style="float:right; font-size:100%; margin-left:15px;" | ||
| |- bgcolor="#efefef" | |- bgcolor="#efefef" | ||
| Line 99: | Line 88: | ||
| ! style="width: 120px" abbr="name" |'''Structure name''' | ! style="width: 120px" abbr="name" |'''Structure name''' | ||
| ! style="width: 130px" abbr="diameter" |'''Structure diameter''' | ! style="width: 130px" abbr="diameter" |'''Structure diameter''' | ||
| ! scope="col" | {{nowrap|{{Abbr|Ref.|Reference}}}} | |||
| |- | |- | ||
| | Cajal bodies || 0.2–2.0 |
| Cajal bodies || 0.2–2.0 μm || <ref name="Cioce">{{cite journal | vauthors = Cioce M, Lamond AI | title = Cajal bodies: a long history of discovery | journal = Annual Review of Cell and Developmental Biology | volume = 21 | pages = 105–31 | year = 2005 | pmid = 16212489 | doi = 10.1146/annurev.cellbio.20.010403.103738 | s2cid = 8807316 | department = Review }}</ref> | ||
| |- | |- | ||
| |Clastosomes | |||
| | PIKA || 5 µm || <ref name="Pollard">{{cite book | |||
| |0.2–0.5 μm | |||
| | last = Pollard | |||
| |<ref name="Lafarga-2002" /> | |||
| | first = Thomas D. | |||
| |author2=William C. Earnshaw | |||
| | title = Cell Biology | |||
| | publisher = Saunders | |||
| | year = 2004 | |||
| | location = Philadelphia | |||
| | isbn = 0-7216-3360-9}}</ref> | |||
| |- | |- | ||
| | PIKA || 5 μm || <ref name="Pollard">{{cite book | last1 = Pollard | first1 = Thomas D. | first2 = William C. | last2 = Earnshaw | name-list-style = vanc | title = Cell Biology | publisher = Saunders | year = 2004 | location = Philadelphia | isbn = 978-0-7216-3360-2 | url-access = registration | url = https://archive.org/details/cellbiology0000poll }}</ref> | |||
| | PML bodies || 0.2–1.0 µm || <ref name="Dundr">{{cite journal | |||
| | last = Dundr | |||
| | first = Miroslav | |||
| |author2=Tom Misteli | |||
| | title = Functional architecture in the cell nucleus | |||
| | journal = Biochem. J. | |||
| | issue = Pt 2 | |||
| | pages = 297–310 | |||
| | year = 2001 | |||
| | pmid = 11368755 | |||
| | volume=356 | |||
| | pmc=1221839 | |||
| | doi=10.1042/0264-6021:3560297 | |||
| }}</ref> | |||
| |- | |- | ||
| | PML bodies || 0.2–1.0 μm || <ref name="Dundr">{{cite journal | vauthors = Dundr M, Misteli T | title = Functional architecture in the cell nucleus | journal = The Biochemical Journal | volume = 356 | issue = Pt 2 | pages = 297–310 | date = June 2001 | pmid = 11368755 | pmc = 1221839 | doi = 10.1042/0264-6021:3560297 | department = Review }}</ref> | |||
| | Paraspeckles || 0.2–1.0 µm || <ref name="rtspara">{{cite interview | |||
| |- | |||
| | last = Fox | |||
| | Paraspeckles || 0.5–1.0 μm || <ref>{{cite journal | vauthors = Bond CS, Fox AH | title = Paraspeckles: nuclear bodies built on long noncoding RNA | journal = The Journal of Cell Biology | volume = 186 | issue = 5 | pages = 637–44 | date = September 2009 | pmid = 19720872 | pmc = 2742191 | doi = 10.1083/jcb.200906113 | department = Review }}</ref> | |||
| | first = Archa | |||
| | interviewer = R. Sundby | |||
| | title =Paraspeckle Size | |||
| | city = E-mail Correspondence | |||
| | date = 2007-03-07 }}</ref> | |||
| |- | |- | ||
| | Speckles || 20–25 nm || <ref name="Pollard" /> | | Speckles || 20–25 nm || <ref name="Pollard" /> | ||
| |} | |} | ||
| Beyond the nuclear bodies first described by ] above (e.g., nucleolus, nuclear speckles, Cajal bodies) the nucleus contains a number of other nuclear bodies. These include polymorphic interphase karyosomal association (PIKA), promyelocytic leukaemia (PML) bodies, and ]s. Although little is known about a number of these domains, they are significant in that they show that the nucleoplasm is not a uniform mixture, but rather contains organized functional subdomains.<ref name="Dundr" /> | |||
| Other subnuclear structures appear as part of abnormal disease processes. For example, the presence of small intranuclear rods has been reported in some cases of ]. This condition typically results from mutations in ], and the rods themselves consist of mutant actin as well as other cytoskeletal proteins.<ref name="Goebel">{{cite |
Other subnuclear structures appear as part of abnormal disease processes. For example, the presence of small intranuclear rods has been reported in some cases of ]. This condition typically results from mutations in ], and the rods themselves consist of mutant actin as well as other cytoskeletal proteins.<ref name="Goebel">{{cite journal | vauthors = Goebel HH, Warlo I | title = Nemaline myopathy with intranuclear rods--intranuclear rod myopathy | journal = Neuromuscular Disorders | volume = 7 | issue = 1 | pages = 13–9 | date = January 1997 | pmid = 9132135 | doi = 10.1016/S0960-8966(96)00404-X | s2cid = 29584217 | department = Review }}</ref> | ||
| ==== |
====PIKA and PTF domains==== | ||
| PIKA domains, or polymorphic interphase karyosomal associations, were first described in microscopy studies in 1991. Their function remains unclear, though they were not thought to be associated with active DNA replication, transcription, or RNA processing.<ref name="Saunders">{{cite journal | vauthors = Saunders WS, Cooke CA, Earnshaw WC | title = Compartmentalization within the nucleus: discovery of a novel subnuclear region | journal = The Journal of Cell Biology | volume = 115 | issue = 4 | pages = 919–31 | date = November 1991 | pmid = 1955462 | pmc = 2289954 | doi = 10.1083/jcb.115.4.919 | department = Primary }}</ref> They have been found to often associate with discrete domains defined by dense localization of the transcription factor PTF, which promotes transcription of ] (snRNA).<ref name="Pombo">{{cite journal | vauthors = Pombo A, Cuello P, Schul W, Yoon JB, Roeder RG, Cook PR, Murphy S | title = Regional and temporal specialization in the nucleus: a transcriptionally-active nuclear domain rich in PTF, Oct1 and PIKA antigens associates with specific chromosomes early in the cell cycle | journal = The EMBO Journal | volume = 17 | issue = 6 | pages = 1768–78 | date = March 1998 | pmid = 9501098 | pmc = 1170524 | doi = 10.1093/emboj/17.6.1768 | department = Primary }}</ref> | |||
| A nucleus typically contains between 1 and 10 compact structures called ] or coiled bodies (CB), whose diameter measures between 0.2 µm and 2.0 µm depending on the cell type and species.<ref name="Cioce" /> When seen under an ], they resemble balls of tangled thread<ref name="Pollard" /> and are dense foci of distribution for the protein ].<ref name="MateraFrey">{{cite journal | author = Matera AG, Frey MA. | title = Coiled Bodies and Gems: Janus or Gemini? | journal = American Journal of Human Genetics | volume = 63 | issue = 2 | pages = 317–321 | year = 1998 | pmid = 9683623 | doi = 10.1086/301992 | pmc=1377332}}</ref> CBs are involved in a number of different roles relating to RNA processing, specifically ] (snoRNA) and ] (snRNA) maturation, and histone mRNA modification.<ref name="Cioce" /> | |||
| ====PML-nuclear bodies==== | |||
| Similar to Cajal bodies are Gemini of coiled bodies, or gems, whose name is derived from the ] in reference to their close "twin" relationship with CBs. Gems are similar in size and shape to CBs, and in fact are virtually indistinguishable under the microscope.<ref name="MateraFrey" /> Unlike CBs, gems do not contain ] (snRNPs), but do contain a protein called survival of motor neuron (SMN) whose function relates to snRNP biogenesis. Gems are believed to assist CBs in snRNP biogenesis,<ref name="Matera">{{cite journal | |||
| ] (PML-nuclear bodies) are spherical bodies found scattered throughout the nucleoplasm, measuring around 0.1–1.0 μm. They are known by a number of other names, including nuclear domain 10 (ND10), Kremer bodies, and PML oncogenic domains.<ref name="Zimber">{{cite journal | vauthors = Zimber A, Nguyen QD, Gespach C | title = Nuclear bodies and compartments: functional roles and cellular signalling in health and disease | journal = Cellular Signalling | volume = 16 | issue = 10 | pages = 1085–104 | date = October 2004 | pmid = 15240004 | doi = 10.1016/j.cellsig.2004.03.020 | department = Review }}</ref> PML-nuclear bodies are named after one of their major components, the promyelocytic leukemia protein (PML). They are often seen in the nucleus in association with Cajal bodies and cleavage bodies.<ref name="Dundr"/> Pml-/- mice, which are unable to create PML-nuclear bodies, develop normally without obvious ill effects, showing that PML-nuclear bodies are not required for most essential biological processes.<ref name="Lallemand2010">{{cite journal | vauthors = Lallemand-Breitenbach V, de Thé H | title = PML nuclear bodies | journal = Cold Spring Harbor Perspectives in Biology | volume = 2 | issue = 5 | pages = a000661 | date = May 2010 | pmid = 20452955 | pmc = 2857171 | doi = 10.1101/cshperspect.a000661 | department = Review }}</ref> | |||
| | last = Matera | |||
| | first = A. Gregory | |||
| | title = Of Coiled Bodies, Gems, and Salmon | |||
| | journal = ] | |||
| | issue = 2 | |||
| | pages = 181–192 | |||
| | year = 1998 | |||
| | pmid = 9671224 | |||
| | accessdate = | doi =10.1002/(sici)1097-4644(19980801)70:2<181::aid-jcb4>3.0.co;2-k | |||
| | volume=70}}</ref> though it has also been suggested from microscopy evidence that CBs and gems are different manifestations of the same structure.<ref name="MateraFrey" /> | |||
| ==== |
====Paraspeckles==== | ||
| {{Main|Paraspeckle}} | |||
| RAFA domains, or polymorphic interphase karyosomal associations, were first described in microscopy studies in 1991. Their function was and remains unclear, though they were not thought to be associated with active DNA replication, transcription, or RNA processing.<ref name="Saunders">{{cite journal | author = Saunders WS, Cooke CA, Earnshaw WC | title = Compartmentalization within the nucleus: discovery of a novel subnuclear region. | journal = ] | volume = 115 | issue = 4 | pages = 919–931 | year = 1991 | doi = 10.1083/jcb.115.4.919 }} PMID 1955462</ref> They have been found to often associate with discrete domains defined by dense localization of the ] PTF, which promotes transcription of ] (snRNA).<ref name="Pombo">{{cite journal | author = Pombo A, Cuello P, Schul W, Yoon J, Roeder R, Cook P, Murphy S | title = Regional and temporal specialization in the nucleus: a transcriptionally active nuclear domain rich in PTF, Oct1 and PIKA antigens associates with specific chromosomes early in the cell cycle | journal = The EMBO Journal| volume = 17 | issue = 6 | pages = 1768–1778 | year = 1998 | pmid = 9501098 | doi = 10.1093/emboj/17.6.1768 | pmc=1170524}}</ref> | |||
| Discovered by Fox et al. in 2002, paraspeckles are irregularly shaped compartments in the interchromatin space of the nucleus.<ref name="Fox_2010">{{cite journal | vauthors = Fox AH, Lamond AI | title = Paraspeckles | journal = Cold Spring Harbor Perspectives in Biology | volume = 2 | issue = 7 | pages = a000687 | date = July 2010 | pmid = 20573717 | pmc = 2890200 | doi = 10.1101/cshperspect.a000687 | department = Review }}</ref> First documented in HeLa cells, where there are generally 10–30 per nucleus,<ref name="para2">{{cite web | last1 =Fox | first1 =Archa | first2 = Wendy | last2 = Bickmore | name-list-style = vanc | title = Nuclear Compartments: Paraspeckles | publisher = Nuclear Protein Database | year = 2004 | url =http://npd.hgu.mrc.ac.uk/compartments/paraspeckles.html | archive-url = http://webarchive.nationalarchives.gov.uk/20080910110920/http://npd.hgu.mrc.ac.uk/compartments/paraspeckles.html | url-status =dead | archive-date =10 September 2008 | access-date = 6 March 2007 }}</ref> paraspeckles are now known to also exist in all human primary cells, transformed cell lines, and tissue sections.<ref name="para3">{{cite journal | vauthors = Fox AH, Bond CS, Lamond AI | title = P54nrb forms a heterodimer with PSP1 that localizes to paraspeckles in an RNA-dependent manner | journal = Molecular Biology of the Cell | volume = 16 | issue = 11 | pages = 5304–15 | date = November 2005 | pmid = 16148043 | pmc = 1266428 | doi = 10.1091/mbc.E05-06-0587 | department = Primary }}</ref> Their name is derived from their distribution in the nucleus; the "para" is short for parallel and the "speckles" refers to the splicing speckles to which they are always in close proximity.<ref name="para2"/> | |||
| Paraspeckles sequester nuclear proteins and RNA and thus appear to function as a molecular sponge<ref name="Nakagawa_2018">{{cite journal | vauthors = Nakagawa S, Yamazaki T, Hirose T | title = Molecular dissection of nuclear paraspeckles: towards understanding the emerging world of the RNP milieu | journal = Open Biology | volume = 8 | issue = 10 | date = October 2018 | page = 180150 | pmid = 30355755 | pmc = 6223218 | doi = 10.1098/rsob.180150 | department = Review }}</ref> that is involved in the regulation of gene expression.<ref name="Pisani_2019">{{cite journal | vauthors = Pisani G, Baron B | title = Nuclear paraspeckles function in mediating gene regulatory and apoptotic pathways | journal = Non-Coding RNA Research | volume = 4 | issue = 4 | pages = 128–134 | date = December 2019 | pmid = 32072080 | pmc = 7012776 | doi = 10.1016/j.ncrna.2019.11.002 | department = Review }}</ref> Furthermore, paraspeckles are dynamic structures that are altered in response to changes in cellular metabolic activity. They are transcription dependent<ref name="Fox_2010" /> and in the absence of RNA Pol II transcription, the paraspeckle disappears and all of its associated protein components (PSP1, p54nrb, PSP2, CFI(m)68, and PSF) form a crescent shaped perinucleolar cap in the nucleolus. This phenomenon is demonstrated during the cell cycle. In the ], paraspeckles are present during ] and during all of ] except for ]. During telophase, when the two daughter nuclei are formed, there is no ] Pol II ] so the protein components instead form a perinucleolar cap.<ref name="para3"/> | |||
| ====PML bodies==== | |||
| Promyelocytic leukaemia bodies (PML bodies) are spherical bodies found scattered throughout the nucleoplasm, measuring around 0.1–1.0 µm. They are known by a number of other names, including nuclear domain 10 (ND10), Kremer bodies, and PML oncogenic domains. PML bodies are named after one of their major components, the ] (PML). They are often seen in the nucleus in association with Cajal bodies and cleavage bodies.<ref name="Dundr"/> PML bodies belong to the ], an ill-defined super-structure of the nucleus proposed to anchor and regulate many nuclear functions, including DNA replication, transcription, or epigenetic silencing.<ref name="Lallemand2010">{{Cite pmid| 20452955}}</ref> The PML protein is the key organizer of these domains that recruits an ever-growing number of proteins, whose only common known feature to date is their ability to be ]. Yet, pml-/- mice (which have their PML gene deleted) cannot assemble nuclear bodies, develop normally and live well, demonstrating that PML bodies are dispensable for most basic biological functions.<ref name="Lallemand2010"/> | |||
| ===={{anchor| |
===={{anchor|Perichromatin fibrils}} Perichromatin fibrils==== | ||
| <!-- This Anchor tag serves to provide a permanent target for incoming section links. Please do not move it out of the section heading, even though it disrupts edit summary generation (you can manually fix the edit summary before saving your changes). Please do not modify it, even if you modify the section title. It is always best to anchor an old section header that has been changed so that links to it won't be broken. See ] for details. (This text: ]) --> | |||
| Speckles are subnuclear structures that are enriched in pre-messenger RNA splicing factors and are located in the interchromatin regions of the nucleoplasm of mammalian cells. At the fluorescence-microscope level they appear as irregular, punctate structures, which vary in size and shape, and when examined by electron microscopy they are seen as clusters of ]. Speckles are dynamic structures, and both their protein and RNA-protein components can cycle continuously between speckles and other nuclear locations, including active transcription sites. Studies on the composition, structure and behaviour of speckles have provided a model for understanding the functional compartmentalization of the nucleus and the organization of the gene-expression machinery<ref>{{cite journal |author=Lamond AI, Spector DL |title=Nuclear speckles: a model for nuclear organelles |journal=Nature Reviews Molecular Cell Biology |volume=4 |issue=8 |pages=605–12 |date=August 2003 |pmid=12923522 |doi=10.1038/nrm1172 |url=}}</ref> | |||
| splicing ]s<ref>{{cite journal |author=Tripathi K, Parnaik VK |title=Differential dynamics of splicing factor SC35 during the cell cycle |journal=J. Biosci. |volume=33 |issue=3 |pages=345–54 |date=September 2008 |pmid=19005234 |doi= 10.1007/s12038-008-0054-3|url=http://www.ias.ac.in/jbiosci/sep2008/345.pdf | format=PDF}}</ref><ref>{{Cite pmid|19005234}}</ref> and other splicing proteins necessary for pre-mRNA processing.<ref>{{cite journal |author=Lamond AI, Spector DL |title=Nuclear speckles: a model for nuclear organelles |journal=Nature Reviews Molecular Cell Biology |volume=4 |issue=8 |pages=605–12 |date=August 2003 |pmid=12923522 |doi=10.1038/nrm1172}}</ref> Because of a cell's changing requirements, the composition and location of these bodies changes according to mRNA transcription and regulation via ] of specific proteins.<ref name="Handwerger">{{cite journal | last =Handwerger | first =Korie E. |author2=Joseph G. Gall | date=January 2006 | title =Subnuclear organelles: new insights into form and function | journal =TRENDS in Cell Biology | volume =16 | issue =1 | pages =19–26 | doi =10.1016/j.tcb.2005.11.005 | pmid = 16325406 }}</ref> | |||
| The splicing speckles are also known as nuclear speckles (nuclear specks), splicing factor compartments (SF compartments), interchromatin granule clusters (IGCs), B snurposomes.<ref> | |||
| {{cite web | |||
| | title = Cellular component Nucleus speckle | |||
| | publisher = UniProt: UniProtKB | |||
| | url = http://www.uniprot.org/locations/SL-0186 | |||
| | accessdate = 2013-08-30}}</ref> | |||
| B snurposomes are found in the amphibian oocyte nuclei and in ''Drosophila melanogaster'' embryos. B snurposomes appear alone or attached to the Cajal bodies in the electron micrographs of the amphibian nuclei.<ref> | |||
| {{Cite journal | |||
| | issn = 1059-1524 | |||
| | volume = 10 | |||
| | issue = 12 | |||
| | pages = 4385–4402 | |||
| | last = Gall | |||
| | first = Joseph G. | |||
| | author2=Bellini, Michel |author3=Wu, Zheng'an |author4=Murphy, Christine | |||
| | title = Assembly of the Nuclear Transcription and Processing Machinery: Cajal Bodies (Coiled Bodies) and Transcriptosomes | |||
| | journal = Molecular Biology of the Cell | |||
| | date = December 1999 | |||
| | pmc=25765 | |||
| | doi = 10.1091/mbc.10.12.4385 | |||
| | pmid = 10588665 | |||
| }}</ref> | |||
| IGCs function as storage sites for the splicing factors.<ref name="Matera2007_NatureMolCellBio"> | |||
| {{Cite journal | |||
| | doi = 10.1038/nrm2124 | |||
| | issn = 1471-0072 | |||
| | volume = 8 | |||
| | issue = 3 | |||
| | pages = 209–220 | |||
| | last = Matera | |||
| | first = A. Gregory | |||
| |author2=Rebecca M. Terns|author3=Michael P. Terns | |||
| | title = Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs | |||
| | journal = Nature Reviews Molecular Cell Biology | |||
| | accessdate = 2013-08-09 | |||
| | date = March 2007 | |||
| | url = http://www.nature.com/nrm/journal/v8/n3/full/nrm2124.html | |||
| | pmid = 17318225 | |||
| }}</ref> | |||
| Perichromatin fibrils are visible only under electron microscope. They are located next to the transcriptionally active chromatin and are hypothesized to be the sites of active ] processing.<ref name="Matera2007_NatureMolCellBio" /> | |||
| ====Paraspeckles==== | |||
| {{main|Paraspeckle}} | |||
| Discovered by Fox et al. in 2002, ]s are irregularly shaped compartments in the nucleus' interchromatin space.<ref name="para1">{{cite journal |author= Fox, Archa |year=2002|url=http://www.current-biology.com/content/article/abstract?uid=PIIS0960982201006327 |title=Paraspeckles:A Novel Nuclear Domain|journal=Current Biology |volume=12 |pages=13–25 |doi=10.1016/S0960-9822(01)00632-7 | pmid=11790299 | last2=Lam | first2=YW | last3=Leung | first3=AK | last4=Lyon | first4=CE | last5=Andersen | first5=J | last6=Mann | first6=M | last7=Lamond | first7=AI | issue=1}}</ref> First documented in HeLa cells, where there are generally 10–30 per nucleus,<ref name="para2">{{cite web | |||
| | last =Fox | |||
| | first =Archa | |||
| |author2=Wendy Bickmore | |||
| | title =Nuclear Compartments: Paraspeckles | |||
| | publisher = Nuclear Protein Database | |||
| | year = 2004 | |||
| | url =http://npd.hgu.mrc.ac.uk/compartments/paraspeckles.html | |||
| | accessdate = 2007-03-06 | archiveurl = http://web.archive.org/web/20060502134554/http://npd.hgu.mrc.ac.uk/compartments/paraspeckles.html| archivedate = May 2, 2006}}</ref> paraspeckles are now known to also exist in all human primary cells, transformed cell lines, and tissue sections.<ref name="para3">{{cite journal |author= Fox, A. |year= 2005 |url= http://www.molbiolcell.org/cgi/reprint/16/11/5304 |title= P54nrb Forms a Heterodimer with PSP1 That Localizes to Paraspeckles in an RNA-dependent Manner | doi = 10.1091/mbc.E05-06-0587 |journal= Molecular Biology of the Cell |volume=16 |pages=5304–5315 | pmid = 16148043 | pmc=1266428 | issue=11|display-authors=etal}}</ref> Their name is derived from their distribution in the nucleus; the "para" is short for parallel and the "speckles" refers to the splicing speckles to which they are always in close proximity.<ref name="para2"/> | |||
| ====Clastosomes==== | |||
| Paraspeckles are dynamic structures that are altered in response to changes in cellular metabolic activity. They are transcription dependent<ref name="para1"/> and in the absence of RNA Pol II transcription, the paraspeckle disappears and all of its associated protein components (PSP1, p54nrb, PSP2, CFI(m)68, and PSF) form a crescent shaped perinucleolar cap in the ]. This phenomenon is demonstrated during the cell cycle. In the ], paraspeckles are present during ] and during all of ] except for ]. During telophase, when the two daughter nuclei are formed, there is no ] Pol II ] so the protein components instead form a perinucleolar cap.<ref name="para3"/> | |||
| Clastosomes are small nuclear bodies (0.2–0.5 μm) described as having a thick ring-shape due to the peripheral capsule around these bodies.<ref name="Lafarga-2002">{{cite journal | vauthors = Lafarga M, Berciano MT, Pena E, Mayo I, Castaño JG, Bohmann D, Rodrigues JP, Tavanez JP, Carmo-Fonseca M | display-authors = 6 | title = Clastosome: a subtype of nuclear body enriched in 19S and 20S proteasomes, ubiquitin, and protein substrates of proteasome | journal = Molecular Biology of the Cell | volume = 13 | issue = 8 | pages = 2771–82 | date = August 2002 | pmid = 12181345 | pmc = 117941 | doi = 10.1091/mbc.e02-03-0122 | citeseerx = 10.1.1.321.6138 | department = Primary }}</ref> This name is derived from the Greek ''klastos'' (]), broken and ''soma'' (]), body.<ref name="Lafarga-2002" /> Clastosomes are not typically present in normal cells, making them hard to detect. They form under high ] conditions within the nucleus and degrade once there is a decrease in activity or if cells are treated with ]s.<ref name="Lafarga-2002" /><ref>{{cite journal | vauthors = Kong XN, Yan HX, Chen L, Dong LW, Yang W, Liu Q, Yu LX, Huang DD, Liu SQ, Liu H, Wu MC, Wang HY | display-authors = 6 | title = LPS-induced down-regulation of signal regulatory protein {alpha} contributes to innate immune activation in macrophages | journal = The Journal of Experimental Medicine | volume = 204 | issue = 11 | pages = 2719–31 | date = October 2007 | pmid = 17954568 | pmc = 2118489 | doi = 10.1084/jem.20062611 | department = Primary }}</ref> The scarcity of clastosomes in cells indicates that they are not required for ] function.<ref name="Carmo-Fonseca-2010">{{cite journal | vauthors = Carmo-Fonseca M, Berciano MT, Lafarga M | title = Orphan nuclear bodies | journal = Cold Spring Harbor Perspectives in Biology | volume = 2 | issue = 9 | pages = a000703 | date = September 2010 | pmid = 20610547 | pmc = 2926751 | doi = 10.1101/cshperspect.a000703 | department = Review }}</ref> ] has also been shown to cause the formation of clastosomes.<ref>{{cite journal | vauthors = Sampuda KM, Riley M, Boyd L | title = Stress induced nuclear granules form in response to accumulation of misfolded proteins in Caenorhabditis elegans | journal = BMC Cell Biology | volume = 18 | issue = 1 | pages = 18 | date = April 2017 | pmid = 28424053 | pmc = 5395811 | doi = 10.1186/s12860-017-0136-x | department = Primary | doi-access = free }}</ref> These nuclear bodies contain catalytic and regulatory subunits of the proteasome and its substrates, indicating that clastosomes are sites for degrading proteins.<ref name="Carmo-Fonseca-2010" /> | |||
| ===={{anchor|Perichromatin fibrils}} Perichromatin fibrils==== | |||
| <!-- This Anchor tag serves to provide a permanent target for incoming section links. Please do not move it out of the section heading, even though it disrupts edit summary generation (you can manually fix the edit summary before saving your changes). Please do not modify it, even if you modify the section title. It is always best to anchor an old section header that has been changed so that links to it won't be broken. See ] for details. (This text: ]) --> | |||
| Perichromatin fibrils are visible only under electron microscope. They are located next to the transcriptionally active chromatin and is hypothesized to be the site of active ] processing.<ref name="Matera2007_NatureMolCellBio" /> | |||
| ==Function== | == Function == | ||
| The nucleus provides a site for |
The nucleus provides a site for ] that is segregated from the location of ] in the cytoplasm, allowing levels of ] that are not available to ]s. The main function of the cell nucleus is to control ] and mediate the ] during the cell cycle.<ref name = "Lodish" />{{rp|171}} | ||
| ===Cell compartmentalization=== | ===Cell compartmentalization=== | ||
| The nuclear envelope allows |
The ] allows control of the nuclear contents, and separates them from the rest of the cytoplasm where necessary. This is important for controlling processes on either side of the nuclear membrane: In most cases where a cytoplasmic process needs to be restricted, a key participant is removed to the nucleus, where it interacts with transcription factors to downregulate the production of certain enzymes in the pathway. This regulatory mechanism occurs in the case of ], a cellular pathway for breaking down ] to produce energy. ] is an enzyme responsible for the first step of glycolysis, forming ] from glucose. At high concentrations of ], a molecule made later from glucose-6-phosphate, a regulator protein removes hexokinase to the nucleus,<ref name="Lehninger">{{cite book | last1 =Lehninger | first1 =Albert L. | last2 =Nelson | first2 =David L. | last3 =Cox | first3 =Michael M. | name-list-style =vanc | title =Lehninger principles of biochemistry | edition =3rd | year =2000 | publisher =Worth Publishers | location =New York | isbn =978-1-57259-931-4 | url-access =registration | url =https://archive.org/details/lehningerprincip01lehn }}</ref> where it forms a transcriptional repressor complex with nuclear proteins to reduce the expression of genes involved in glycolysis.<ref name="Moreno">{{cite journal | vauthors = Moreno F, Ahuatzi D, Riera A, Palomino CA, Herrero P | title = Glucose sensing through the Hxk2-dependent signalling pathway | journal = Biochemical Society Transactions | volume = 33 | issue = Pt 1 | pages = 265–8 | date = February 2005 | pmid = 15667322 | doi = 10.1042/BST0330265 | s2cid = 20647022 | department = Primary }}</ref> | ||
| In order to control which genes are being transcribed, the cell separates some |
In order to control which genes are being transcribed, the cell separates some transcription factor proteins responsible for regulating gene expression from physical access to the DNA until they are activated by other signaling pathways. This prevents even low levels of inappropriate gene expression. For example, in the case of ]-controlled genes, which are involved in most ] responses, transcription is induced in response to a ] such as that initiated by the signaling molecule ], binds to a cell membrane receptor, resulting in the recruitment of signalling proteins, and eventually activating the transcription factor NF-κB. A ] on the NF-κB protein allows it to be transported through the nuclear pore and into the nucleus, where it stimulates the transcription of the target genes.<ref name="MBoC" /> | ||
| The compartmentalization allows the cell to prevent translation of unspliced mRNA.<ref name="Gorlich">{{cite |
The compartmentalization allows the cell to prevent translation of unspliced mRNA.<ref name="Gorlich">{{cite journal | vauthors = Görlich D, Kutay U | title = Transport between the cell nucleus and the cytoplasm | journal = Annual Review of Cell and Developmental Biology | volume = 15 | issue = 1 | pages = 607–60 | year = 1999 | pmid = 10611974 | doi = 10.1146/annurev.cellbio.15.1.607 | department = Review }}</ref> Eukaryotic mRNA contains introns that must be removed before being translated to produce functional proteins. The splicing is done inside the nucleus before the mRNA can be accessed by ribosomes for translation. Without the nucleus, ribosomes would translate newly transcribed (unprocessed) mRNA, resulting in malformed and nonfunctional proteins.<ref name = "Lodish" />{{rp|108–15}} | ||
| === |
===Replication=== | ||
| {{Main|Eukaryotic DNA replication}} | |||
| {{main|Gene expression}} | |||
| The main function of the cell nucleus is to control gene expression and mediate the replication of DNA during the cell cycle.<ref name = "Lodish" />{{rp|171}} It has been found that replication happens in a localised way in the cell nucleus. In the S phase of interphase of the cell cycle; replication takes place. Contrary to the traditional view of moving replication forks along stagnant DNA, a concept of ''replication factories'' emerged, which means replication forks are concentrated towards some immobilised 'factory' regions through which the template DNA strands pass like conveyor belts.<ref name="Hozák_1994">{{cite journal | vauthors = Hozák P, Cook PR | title = Replication factories | journal = Trends in Cell Biology | volume = 4 | issue = 2 | pages = 48–52 | date = February 1994 | pmid = 14731866 | doi = 10.1016/0962-8924(94)90009-4 | department = Review }}</ref> | |||
| ] of ] illustrating the growing ]s. "Begin" indicates the ] of the DNA, where new RNA synthesis begins; "end" indicates the ], where the primary transcripts are almost complete.]] | |||
| ===Gene expression=== | |||
| Gene expression first involves ], in which DNA is used as a template to produce RNA. In the case of genes encoding proteins, that RNA produced from this process is ] (mRNA), which then needs to be ] by ] to form a protein. As ribosomes are located outside the nucleus, mRNA produced needs to be exported.<ref>{{cite book |title=Protein Synthesis and Ribosome Structure: Translating the Genome |last=Nierhaus |first=Knud H. |author2=Daniel N. Wilson |year=2004 |publisher=Wiley-VCH |isbn=3-527-30638-2 }}</ref> | |||
| {{Main|Gene expression}} | |||
| {{See also|Transcription factories}} | |||
| ] during transcription, highlighting the possibility of transcribing more than one gene at a time. The diagram includes 8 RNA polymerases however the number can vary depending on cell type. The image also includes transcription factors and a porous, protein core.]] | |||
| Gene expression first involves transcription, in which DNA is used as a template to produce RNA. In the case of genes encoding proteins, that RNA produced from this process is messenger RNA (mRNA), which then needs to be translated by ribosomes to form a protein. As ribosomes are located outside the nucleus, mRNA produced needs to be exported.<ref>{{cite book |title=Protein Synthesis and Ribosome Structure: Translating the Genome |last1=Nierhaus |first1=Knud H. | first2 = Daniel N. | last2 = Wilson | name-list-style = vanc |year=2004 |publisher=Wiley-VCH |isbn=978-3-527-30638-1 }}</ref> | |||
| Since the nucleus is the site of transcription, it also contains a variety of proteins that either directly mediate transcription or are involved in regulating the process. These proteins include ]s, which unwind the double-stranded DNA molecule to facilitate access to it, ]s, which synthesize the growing RNA molecule, ]s, which change the amount of ]ing in DNA, helping it wind and unwind, as well as a large variety of ]s that regulate expression.<ref>{{cite book |title=Genome Structure and Function: From Chromosomes Characterization to Genes Technology |last=Nicolini |first=Claudio A. |year=1997 |publisher=Springer |isbn=0-7923-4565-7 }}</ref> | |||
| Since the nucleus is the site of transcription, it also contains a variety of proteins that either directly mediate transcription or are involved in regulating the process. These proteins include ]s, which unwind the double-stranded DNA molecule to facilitate access to it, ]s, which bind to the DNA promoter to synthesize the growing RNA molecule, ]s, which change the amount of ]ing in DNA, helping it wind and unwind, as well as a large variety of transcription factors that regulate expression.<ref>{{cite book |title=Genome Structure and Function: From Chromosomes Characterization to Genes Technology |last=Nicolini |first=Claudio A. | name-list-style = vanc |year=1997 |publisher=Springer |isbn=978-0-7923-4565-7 }}</ref> | |||
| ===Processing of pre-mRNA=== | ===Processing of pre-mRNA=== | ||
| {{ |
{{Main|Post-transcriptional modification}} | ||
| Newly synthesized mRNA molecules are known as ]s or pre-mRNA. They must undergo ] in the nucleus before being exported to the cytoplasm; mRNA that appears in the cytoplasm without these modifications is degraded rather than used for protein |
Newly synthesized mRNA molecules are known as ]s or pre-mRNA. They must undergo ] in the nucleus before being exported to the cytoplasm; mRNA that appears in the cytoplasm without these modifications is degraded rather than used for protein translation. The three main modifications are ]ping, 3' ], and ]. While in the nucleus, pre-mRNA is associated with a variety of proteins in complexes known as ]s (hnRNPs). Addition of the 5' cap occurs co-transcriptionally and is the first step in post-transcriptional modification. The 3' poly-] tail is only added after transcription is complete.<ref name = "Lodish" />{{rp|509–18}} | ||
| RNA splicing, carried out by a complex called the ], is the process by which |
RNA splicing, carried out by a complex called the ], is the process by which introns, or regions of DNA that do not code for protein, are removed from the pre-mRNA and the remaining ]s connected to re-form a single continuous molecule. This process normally occurs after 5' capping and 3' polyadenylation but can begin before synthesis is complete in transcripts with many exons.<ref name="Lodish" />{{rp|494}} Many pre-mRNAs can be spliced in multiple ways to produce different mature mRNAs that encode different ]. This process is known as ], and allows production of a large variety of proteins from a limited amount of DNA.<ref name=Black>{{cite journal | vauthors = Black DL | title = Mechanisms of alternative pre-messenger RNA splicing | journal = Annual Review of Biochemistry | volume = 72 | issue = 1 | pages = 291–336 | year = 2003 | pmid = 12626338 | doi = 10.1146/annurev.biochem.72.121801.161720 | s2cid = 23576288 | url = https://cloudfront.escholarship.org/dist/prd/content/qt2hg605wm/qt2hg605wm.pdf | department = Review }}</ref> | ||
| {{ |
{{Clear}} | ||
| ==Dynamics and regulation== | ==Dynamics and regulation== | ||
| ===Nuclear transport=== | ===Nuclear transport=== | ||
| {{ |
{{Main|Nuclear transport}} | ||
| ]s, such as ] and ]s, are ] across the nuclear membrane in a process called the ]-] nuclear transport cycle.]] | ]s, such as ] and ]s, are ] across the nuclear membrane in a process called the ]-] nuclear transport cycle.]] | ||
| The entry and exit of large molecules from the nucleus is tightly controlled by the nuclear pore complexes. Although small molecules can enter the nucleus without regulation,<ref name="Watson">{{cite book | |
The entry and exit of large molecules from the nucleus is tightly controlled by the nuclear pore complexes. Although small molecules can enter the nucleus without regulation,<ref name="Watson">{{cite book | vauthors = Watson JD, Baker TA, Bell SP, Gann A, Levine M, Losick R | title = Molecular Biology of the Gene | publisher = Peason Benjamin Cummings; CSHL Press. | year = 2004 | edition = 5th | chapter = Ch9–10 | isbn = 978-0-8053-9603-4 }}</ref> macromolecules such as RNA and proteins require association karyopherins called ]s to enter the nucleus and ]s to exit. "Cargo" proteins that must be translocated from the cytoplasm to the nucleus contain short amino acid sequences known as ]s, which are bound by importins, while those transported from the nucleus to the cytoplasm carry ]s bound by exportins. The ability of importins and exportins to transport their cargo is regulated by ]s, enzymes that ] the molecule guanosine triphosphate (GTP) to release energy. The key GTPase in nuclear transport is ], which is bound to either GTP or GDP (guanosine diphosphate), depending on whether it is located in the nucleus or the cytoplasm. Whereas importins depend on RanGTP to dissociate from their cargo, exportins require RanGTP in order to bind to their cargo.<ref name="Pemberton" /> | ||
| Nuclear import depends on the importin binding its cargo in the cytoplasm and carrying it through the nuclear pore into the nucleus. Inside the nucleus, RanGTP acts to separate the cargo from the importin, allowing the importin to exit the nucleus and be reused. Nuclear export is similar, as the exportin binds the cargo inside the nucleus in a process facilitated by RanGTP, exits through the nuclear pore, and separates from its cargo in the cytoplasm. | Nuclear import depends on the importin binding its cargo in the cytoplasm and carrying it through the nuclear pore into the nucleus. Inside the nucleus, RanGTP acts to separate the cargo from the importin, allowing the importin to exit the nucleus and be reused. Nuclear export is similar, as the exportin binds the cargo inside the nucleus in a process facilitated by RanGTP, exits through the nuclear pore, and separates from its cargo in the cytoplasm.<ref name="Cavazza_2015">{{cite journal | vauthors = Cavazza T, Vernos I | title = The RanGTP Pathway: From Nucleo-Cytoplasmic Transport to Spindle Assembly and Beyond | journal = Frontiers in Cell and Developmental Biology | volume = 3 | pages = 82 | date = 2015 | pmid = 26793706 | pmc = 4707252 | doi = 10.3389/fcell.2015.00082 | department = Review | doi-access = free }}</ref> | ||
| Specialized export proteins exist for translocation of mature mRNA and tRNA to the cytoplasm after post-transcriptional modification is complete. This quality-control mechanism is important due to these molecules' central role in protein translation |
Specialized export proteins exist for translocation of mature mRNA and tRNA to the cytoplasm after post-transcriptional modification is complete. This quality-control mechanism is important due to these molecules' central role in protein translation. Mis-expression of a protein due to incomplete excision of exons or mis-incorporation of amino acids could have negative consequences for the cell; thus, incompletely modified RNA that reaches the cytoplasm is degraded rather than used in translation.<ref name="Lodish" /> | ||
| ===Assembly and disassembly=== | ===Assembly and disassembly=== | ||
| ] ] cell ] with ] ]s during ]. The ] can be seen, stained green, attached to the two sets of ]s, stained light blue. All chromosomes but one are already at the metaphase plate. ]] | ] ] cell ] with ] ]s during ]. The ] can be seen, stained green, attached to the two sets of ]s, stained light blue. All chromosomes but one are already at the metaphase plate. ]] | ||
| During its lifetime, a nucleus may be broken down, either in the process of ] or as a consequence of ] (the process of ]). During these events, the structural components of the nucleus — the envelope and lamina — can be systematically degraded. | During its lifetime, a nucleus may be broken down or destroyed, either in the process of ] or as a consequence of ] (the process of ]). During these events, the structural components of the nucleus — the envelope and lamina — can be systematically degraded. | ||
| In most cells, the disassembly of the nuclear envelope marks the end of the ] of |
In most cells, the disassembly of the nuclear envelope marks the end of the ] of mitosis. However, this disassembly of the nucleus is not a universal feature of mitosis and does not occur in all cells. Some unicellular eukaryotes (e.g., yeasts) undergo so-called ], in which the nuclear envelope remains intact. In closed mitosis, the daughter chromosomes migrate to opposite poles of the nucleus, which then divides in two. The cells of higher eukaryotes, however, usually undergo ], which is characterized by breakdown of the nuclear envelope. The daughter chromosomes then migrate to opposite poles of the mitotic spindle, and new nuclei reassemble around them.<ref name = "Lodish" />{{rp|854}} | ||
| At a certain point during the |
At a certain point during the cell cycle in open mitosis, the cell divides to form two cells. In order for this process to be possible, each of the new daughter cells must have a full set of genes, a process requiring replication of the chromosomes as well as segregation of the separate sets. This occurs by the replicated chromosomes, the ]s, attaching to ]s, which in turn are attached to different ]s. The sister chromatids can then be pulled to separate locations in the cell. In many cells, the centrosome is located in the cytoplasm, outside the nucleus; the microtubules would be unable to attach to the chromatids in the presence of the nuclear envelope.<ref name="Lippincott-Schwartz">{{cite journal | vauthors = Lippincott-Schwartz J | title = Cell biology: ripping up the nuclear envelope | journal = Nature | volume = 416 | issue = 6876 | pages = 31–2 | date = March 2002 | pmid = 11882878 | doi = 10.1038/416031a | url = https://zenodo.org/record/1233215 | s2cid = 4431000 | department = Commentary | bibcode = 2002Natur.416...31L | doi-access = free }}</ref> Therefore, the early stages in the cell cycle, beginning in prophase and until around ], the nuclear membrane is dismantled.<ref name="RGoldman" /> Likewise, during the same period, the nuclear lamina is also disassembled, a process regulated by phosphorylation of the lamins by protein kinases such as the ].<ref name="Boulikas">{{cite journal | vauthors = Boulikas T | title = Phosphorylation of transcription factors and control of the cell cycle | journal = Critical Reviews in Eukaryotic Gene Expression | volume = 5 | issue = 1 | pages = 1–77 | year = 1995 | pmid = 7549180 | department = Review }}</ref> Towards the end of the cell cycle, the nuclear membrane is reformed, and around the same time, the nuclear lamina are reassembled by dephosphorylating the lamins.<ref name="Boulikas" /> | ||
| However, in ], the nuclear envelope remains intact, the centrosomes are located in the cytoplasm, and the microtubules come in contact with chromosomes, whose centromeric regions are incorporated into the nuclear envelope (the so-called closed mitosis with extranuclear spindle). In many other protists (e.g., ]s, ]) and fungi, the centrosomes are intranuclear, and their nuclear envelope also does not |
However, in ], the nuclear envelope remains intact, the centrosomes are located in the cytoplasm, and the microtubules come in contact with chromosomes, whose centromeric regions are incorporated into the nuclear envelope (the so-called closed mitosis with extranuclear spindle). In many other protists (e.g., ]s, ]) and fungi, the centrosomes are intranuclear, and their nuclear envelope also does not disassemble during cell division.<ref name="Boettcher_2013">{{cite journal | vauthors = Boettcher B, Barral Y | title = The cell biology of open and closed mitosis | journal = Nucleus | location = Austin, Tex. | volume = 4 | issue = 3 | pages = 160–5 | date = 2013 | pmid = 23644379 | pmc = 3720745 | doi = 10.4161/nucl.24676 | department = Review }}</ref> | ||
| Apoptosis is a controlled process in which the cell's structural components are destroyed, resulting in death of the cell. Changes associated with apoptosis directly affect the nucleus and its contents, for example, in the condensation of chromatin and the disintegration of the nuclear envelope and lamina. The destruction of the lamin networks is controlled by specialized apoptotic ]s called ]s, which cleave the lamin proteins and, thus, degrade the nucleus' structural integrity. Lamin cleavage is sometimes used as a laboratory indicator of caspase activity in ]s for early apoptotic activity.<ref name="RGoldman" /> Cells that express mutant caspase-resistant lamins are deficient in nuclear changes related to apoptosis, suggesting that lamins play a role in initiating the events that lead to apoptotic degradation of the nucleus.<ref name="RGoldman" /> Inhibition of lamin assembly itself is an inducer of apoptosis.<ref name="Steen">{{cite journal | vauthors = Steen RL, Collas P | title = Mistargeting of B-type lamins at the end of mitosis: implications on cell survival and regulation of lamins A/C expression | journal = The Journal of Cell Biology | volume = 153 | issue = 3 | pages = 621–6 | date = April 2001 | pmid = 11331311 | pmc = 2190567 | doi = 10.1083/jcb.153.3.621 | department = Primary }}</ref> | |||
| The nuclear envelope acts as a barrier that prevents both DNA and RNA viruses from entering the nucleus. Some viruses require access to proteins inside the nucleus in order to replicate and/or assemble. DNA viruses, such as ] replicate and assemble in the cell nucleus, and exit by budding through the inner nuclear membrane. This process is accompanied by disassembly of the lamina on the nuclear face of the inner membrane.<ref name="RGoldman" /> | The nuclear envelope acts as a barrier that prevents both DNA and RNA viruses from entering the nucleus. Some viruses require access to proteins inside the nucleus in order to replicate and/or assemble. DNA viruses, such as ] replicate and assemble in the cell nucleus, and exit by budding through the inner nuclear membrane. This process is accompanied by disassembly of the lamina on the nuclear face of the inner membrane.<ref name="RGoldman" /> | ||
| ===Disease-related dynamics=== | ===Disease-related dynamics=== | ||
| Initially, it has been suspected that ] in general and ] in particular do not enter the nucleus. Now there is a body of evidence that under pathological conditions (e.g. ]) IgG can enter the nucleus.<ref>Böhm I |
Initially, it has been suspected that ] in general and ] in particular do not enter the nucleus. Now there is a body of evidence that under pathological conditions (e.g. ]) IgG can enter the nucleus.<ref name="pmid17364135">{{cite journal | vauthors = Böhm I | title = IgG deposits can be detected in cell nuclei of patients with both lupus erythematosus and malignancy | journal = Clinical Rheumatology | volume = 26 | issue = 11 | pages = 1877–82 | date = November 2007 | pmid = 17364135 | doi = 10.1007/s10067-007-0597-y | s2cid = 44879431 | department = Primary }}</ref> | ||
| ==Nuclei per cell== | ==Nuclei per cell== | ||
| Most ] cell types usually have a single nucleus, but some have no nuclei, while others have several. This can result from normal development, as in the maturation of mammalian ]s, or from faulty cell division. | Most ] cell types usually have a single nucleus, but some have no nuclei, while others have several. This can result from normal development, as in the maturation of mammalian ]s, or from faulty cell division.<ref>{{cite book |last1=Ressel |first1=Lorenzo | name-list-style = vanc | chapter = Nuclear Morphologies |title=Normal cell morphology in canine and feline cytology: an identification guide |date=2017 |publisher=John Wiley & Sons |location=Hoboken, NJ |isbn=978-1-119-27891-7 |page=6 | chapter-url=https://books.google.com/books?id=AkwrDwAAQBAJ&pg=PA6}}</ref> | ||
| ===Anucleated cells=== | ===Anucleated cells=== | ||
| Line 288: | Line 197: | ||
| ] | ] | ||
| An anucleated cell contains no nucleus and is, therefore, incapable of dividing to produce daughter cells. The best-known anucleated cell is the mammalian red blood cell, or ], which also lacks other organelles such as mitochondria, and serves primarily as a transport vessel to ferry ] from the ] to the body's tissues. Erythrocytes mature through ] in the ], where they lose their nuclei, organelles, and ribosomes. The nucleus is expelled during the process of differentiation from an ] to a ], which is the immediate precursor of the mature erythrocyte.<ref name="Skutelsky">{{cite journal | vauthors = Skutelsky E, Danon D | title = Comparative study of nuclear expulsion from the late erythroblast and cytokinesis | journal = Experimental Cell Research | volume = 60 | issue = 3 | pages = 427–36 | date = June 1970 | pmid = 5422968 | doi = 10.1016/0014-4827(70)90536-7 | department = Primary }}</ref> The presence of ]s may induce the release of some immature "micronucleated" erythrocytes into the bloodstream.<ref name="Torous">{{cite journal | vauthors = Torous DK, Dertinger SD, Hall NE, Tometsko CR | title = Enumeration of micronucleated reticulocytes in rat peripheral blood: a flow cytometric study | journal = Mutation Research | volume = 465 | issue = 1–2 | pages = 91–9 | date = February 2000 | pmid = 10708974 | doi = 10.1016/S1383-5718(99)00216-8 | department = Primary }}</ref><ref name="Hutter">{{cite journal | vauthors = Hutter KJ, Stöhr M | title = Rapid detection of mutagen induced micronucleated erythrocytes by flow cytometry | journal = Histochemistry | volume = 75 | issue = 3 | pages = 353–62 | year = 1982 | pmid = 7141888 | doi = 10.1007/bf00496738 | s2cid = 28973947 | department = Primary }}</ref> Anucleated cells can also arise from flawed cell division in which one daughter lacks a nucleus and the other has two nuclei. | |||
| In ]s, this condition occurs in ]s.<ref>{{cite journal | vauthors = Ham BK, Lucas WJ | title = The angiosperm phloem sieve tube system: a role in mediating traits important to modern agriculture | journal = Journal of Experimental Botany | volume = 65 | issue = 7 | pages = 1799–816 | date = April 2014 | pmid = 24368503 | doi = 10.1093/jxb/ert417 | doi-access = free }}</ref> | |||
| ===Multinucleated cells=== | ===Multinucleated cells=== | ||
| {{Main|Multinucleate}} | |||
| ] cells contain multiple nuclei. Most ]n species of ]<ref name="Zettler">{{cite journal | vauthors = Zettler LA, Sogin ML, Caron DA | title = Phylogenetic relationships between the Acantharea and the Polycystinea: a molecular perspective on Haeckel's Radiolaria | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 94 | issue = 21 | pages = 11411–6 | date = October 1997 | pmid = 9326623 | pmc = 23483 | doi = 10.1073/pnas.94.21.11411 | department = Primary | bibcode = 1997PNAS...9411411A | doi-access = free }}</ref> and some ] in ]<ref name="Horton">{{cite journal | vauthors = Horton TR | title = The number of nuclei in basidiospores of 63 species of ectomycorrhizal Homobasidiomycetes | journal = Mycologia | volume = 98 | issue = 2 | pages = 233–8 | year = 2006 | pmid = 16894968 | doi = 10.3852/mycologia.98.2.233 | department = Primary }}</ref> have naturally multinucleated cells. Other examples include the ]s in the genus '']'', which have two nuclei per cell.<ref>{{cite journal | vauthors = Adam RD | title = The biology of Giardia spp | journal = Microbiological Reviews | volume = 55 | issue = 4 | pages = 706–32 | date = December 1991 | pmid = 1779932 | pmc = 372844 | doi = 10.1128/MMBR.55.4.706-732.1991 | department = Review }}</ref> ]s have two kinds of nuclei in a single cell, a somatic ] and a germline ].<ref>{{cite journal |vauthors=Vogt A, Goldman AD, Mochizuki K, Landweber LF |title=Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements |journal=PLOS Genetics |date=1 August 2013 |volume=9 |issue=8 |pages=e1003659 |doi=10.1371/journal.pgen.1003659 |pmid=23935529 |pmc=3731211 |doi-access=free }}</ref> In humans, ], also called ]s and ], become multinucleated during development; the resulting arrangement of nuclei near the periphery of the cells allows maximal intracellular space for ].<ref name="Lodish" /> Other multinucleate cells in the human are ]s a type of ]. Multinucleated and ] can also be abnormal in humans; for example, cells arising from the fusion of ]s and ]s, known as ]s, sometimes accompany inflammation<ref name="McInnes">{{cite journal | vauthors = McInnes A, Rennick DM | title = Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells | journal = The Journal of Experimental Medicine | volume = 167 | issue = 2 | pages = 598–611 | date = February 1988 | pmid = 3258008 | pmc = 2188835 | doi = 10.1084/jem.167.2.598 | department = Primary }}</ref> and are also implicated in tumor formation.<ref name="Goldring">{{cite journal | vauthors = Goldring SR, Roelke MS, Petrison KK, Bhan AK | title = Human giant cell tumors of bone identification and characterization of cell types | journal = The Journal of Clinical Investigation | volume = 79 | issue = 2 | pages = 483–91 | date = February 1987 | pmid = 3027126 | pmc = 424109 | doi = 10.1172/JCI112838 | department = Primary }}</ref> | |||
| A number of ]s are known to have two nuclei. Unlike other multinucleated cells these nuclei contain two distinct lineages of DNA: one from the dinoflagellate and the other from a symbiotic ].<ref name=Imanian2012>{{cite journal | vauthors = Imanian B, Pombert JF, Dorrell RG, Burki F, Keeling PJ | title = Tertiary endosymbiosis in two dinotoms has generated little change in the mitochondrial genomes of their dinoflagellate hosts and diatom endosymbionts | journal = PLOS ONE | volume = 7 | issue = 8 | pages = e43763 | year = 2012 | pmid = 22916303 | pmc = 3423374 | doi = 10.1371/journal.pone.0043763 | bibcode = 2012PLoSO...743763I | department = Primary | doi-access = free }}</ref> | |||
| ] cells contain multiple nuclei. Most ]n species of ]<ref name="Zettler">{{cite journal | last =Zettler | first =LA |author2=Sogin ML|author3=Caron DA | date = | year =1997 | title =Phylogenetic relationships between the Acantharea and the Polycystinea: A molecular perspective on Haeckel's Radiolaria | journal =Proc Natl Acad Sci USA | volume =94 | issue=21 | pages =11411–11416 | pmid =9326623 | url = | doi = 10.1073/pnas.94.21.11411 | pmc=23483 }}</ref> and some ] in ]<ref name="Horton">{{cite journal | last =Horton | first =TR | year =2006 | title =The number of nuclei in basidiospores of 63 species of ectomycorrhizal Homobasidiomycetes | journal =Mycologia | volume =98 |issue=2 | pages =233–238 | pmid =16894968 | doi = 10.3852/mycologia.98.2.233}}</ref> have naturally multinucleated cells. Other examples include the ]s in the genus '']'', which have two nuclei per cell.<ref>{{cite journal |author=Adam RD |title=The biology of Giardia spp |journal=Microbiol. Rev. |volume=55 |issue=4 |pages=706–32 |date=December 1991 |pmid=1779932 |pmc=372844 |url=http://mmbr.asm.org/cgi/pmidlookup?view=long&pmid=1779932}}</ref> In humans, ] cells, called ]s and ], become multinucleated during development; the resulting arrangement of nuclei near the periphery of the cells allows maximal intracellular space for ].<ref name="Lodish" /> Multinucleated and ] can also be abnormal in humans; for example, cells arising from the fusion of ]s and ]s, known as ]s, sometimes accompany inflammation<ref name="McInnes">{{cite journal | last =McInnes | first =A |author2=Rennick DM | date = | year =1988 | title =Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells | journal =J Exp Med | issue =2 | pages =598–611 | pmid =3258008 | doi =10.1084/jem.167.2.598 | volume=167 | pmc=2188835}}</ref> and are also implicated in tumor formation.<ref name="Goldring">{{cite journal | last =Goldring | first =SR |author2=Roelke MS|author3=Petrison KK|author4=Bhan AK | year =1987 | title =Human giant cell tumors of bone identification and characterization of cell types | journal =J Clin Invest | volume =79 |issue=2 | pages =483–491 | pmid =3027126 | doi = 10.1172/JCI112838 | pmc=424109}}</ref> | |||
| A number of ]s are known to have two nuclei.<ref name=Imanian2012>{{cite journal | last1 = Imanian | first1 = B | last2 = Pombert | first2 = JF | last3 = Dorrell | first3 = RG | last4 = Burki | first4 = F | last5 = Keeling | first5 = PJ | year = 2012 | title = Tertiary endosymbiosis in two dinotoms has generated little change in the mitochondrial genomes of their dinoflagellate hosts and diatom endosymbionts | url = | journal = PLOS ONE | volume = 7 | issue = 8| page = e43763 | doi=10.1371/journal.pone.0043763}}</ref> Unlike other multinucleated cells these nuclei contain two distinct lineages of DNA: one from the dinoflagelate and the other from a symbiotic ]. Curiously the ] and the ] of the diatom remain functional. | |||
| ==Evolution== | ==Evolution== | ||
| As the major defining characteristic of the eukaryotic cell, the nucleus' ]ary origin has been the subject of much speculation. Four major hypotheses have been proposed to explain the existence of the nucleus, although none have yet earned widespread support.<ref name="Pennisi">{{cite journal | |
As the major defining characteristic of the eukaryotic cell, the nucleus's ]ary origin has been the subject of much speculation. Four major hypotheses have been proposed to explain the existence of the nucleus, although none have yet earned widespread support.<ref name="Pennisi">{{cite journal | vauthors = Pennisi E | author-link = Elizabeth Pennisi | title = Evolutionary biology. The birth of the nucleus | journal = Science | volume = 305 | issue = 5685 | pages = 766–8 | date = August 2004 | pmid = 15297641 | doi = 10.1126/science.305.5685.766 | s2cid = 83769250 | department = News }}</ref><ref name="Devos_2014">{{cite journal | vauthors = Devos DP, Gräf R, Field MC | title = Evolution of the nucleus | journal = Current Opinion in Cell Biology | volume = 28 | pages = 8–15 | date = June 2014 | issue = 100 | pmid = 24508984 | pmc = 4071446 | doi = 10.1016/j.ceb.2014.01.004 | department = Review }}</ref><ref name="López-García_2015">{{cite journal | vauthors = López-García P, Moreira D | title = Open Questions on the Origin of Eukaryotes | journal = Trends in Ecology & Evolution | volume = 30 | issue = 11 | pages = 697–708 | date = November 2015 | pmid = 26455774 | pmc = 4640172 | doi = 10.1016/j.tree.2015.09.005 | department = Review }}</ref> | ||
| The first model known as the "syntrophic model" proposes that a ] relationship between the ] and ] created the nucleus-containing eukaryotic cell. (Organisms of the |
The first model known as the "syntrophic model" proposes that a ] relationship between the ] and ] created the nucleus-containing eukaryotic cell. (Organisms of the Archaeal and Bacterial domains have no cell nucleus.<ref>{{cite book | vauthors = Hogan CM | chapter = Archaea | title = Encyclopedia of Earth | veditors = Monosson E, Cleveland C | publisher = National Council for Science and the Environment | location = Washington, DC. | date = 2010 | archive-url = https://web.archive.org/web/20110511133400/http://www.eoearth.org/article/Archaea?topic=49496 | archive-date = 11 May 2011 | chapter-url = http://www.eoearth.org/article/Archaea?topic=49496 }}</ref>) It is hypothesized that the symbiosis originated when ancient archaea similar to modern ] archaea, invaded and lived within bacteria similar to modern ], eventually forming the early nucleus. This theory is analogous to the accepted theory for the origin of eukaryotic mitochondria and ]s, which are thought to have developed from a similar endosymbiotic relationship between proto-eukaryotes and aerobic bacteria.<ref name="Margulis">{{cite book | last= Margulis | first= Lynn | author-link= Lynn Margulis |name-list-style= vanc | year= 1981 | title= Symbiosis in Cell Evolution | pages= | publisher= W. H. Freeman and Company | location= San Francisco | isbn= 978-0-7167-1256-5 | url= https://archive.org/details/symbiosisincelle00marg/page/206 }}</ref> One possibility is that the nuclear membrane arose as a new membrane system following the origin of ] in an ] host.<ref name="Martin">{{cite journal |vauthors=Martin W |title=Archaebacteria (Archaea) and the origin of the eukaryotic nucleus |journal=Curr Opin Microbiol |volume=8 |issue=6 |pages=630–7 |date=December 2005 |pmid=16242992 |doi=10.1016/j.mib.2005.10.004 |url=}}</ref> The nuclear membrane may have served to protect the genome from damaging ] produced by the protomitochondria.<ref>Bernstein, H., Bernstein, C. (2017). Sexual Communication in Archaea, the Precursor to Eukaryotic Meiosis. In: Witzany, G. (eds) Biocommunication of Archaea. Springer, Cham. https://doi.org/10.1007/978-3-319-65536-9_7</ref> The archaeal origin of the nucleus is supported by observations that archaea and eukarya have similar genes for certain proteins, including ]s. Observations that myxobacteria are motile, can form multicellular complexes, and possess ]s and ]s similar to eukarya, support a bacterial origin for the eukaryotic cell.<ref name="Lopez-Garcia">{{cite journal | vauthors = López-García P, Moreira D | title = Selective forces for the origin of the eukaryotic nucleus | journal = BioEssays | volume = 28 | issue = 5 | pages = 525–33 | date = May 2006 | pmid = 16615090 | doi = 10.1002/bies.20413 | department = Review }}</ref> | ||
| A second model proposes that proto-eukaryotic cells evolved from bacteria without an endosymbiotic stage. This model is based on the existence of modern ] bacteria that possess a nuclear structure with primitive pores and other compartmentalized membrane structures.<ref name="Fuerst">{{cite |
A second model proposes that proto-eukaryotic cells evolved from bacteria without an endosymbiotic stage. This model is based on the existence of modern ] bacteria that possess a nuclear structure with primitive pores and other compartmentalized membrane structures.<ref name="Fuerst">{{cite journal | vauthors = Fuerst JA | title = Intracellular compartmentation in planctomycetes | journal = Annual Review of Microbiology | volume = 59 | pages = 299–328 | year = 2005 | pmid = 15910279 | doi = 10.1146/annurev.micro.59.030804.121258 | department = Review }}</ref> A similar proposal states that a eukaryote-like cell, the ], evolved first and ] archaea and bacteria to generate the nucleus and the eukaryotic cell.<ref name="Hartman">{{cite journal | vauthors = Hartman H, Fedorov A | title = The origin of the eukaryotic cell: a genomic investigation | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 99 | issue = 3 | pages = 1420–5 | date = February 2002 | pmid = 11805300 | pmc = 122206 | doi = 10.1073/pnas.032658599 | department = Primary | bibcode = 2002PNAS...99.1420H | doi-access = free }}</ref> | ||
| The most controversial model, known as '']'', posits that the membrane-bound nucleus, along with other eukaryotic features, originated from the infection of a prokaryote by a virus. The suggestion is based on similarities between eukaryotes and viruses such as linear DNA strands, mRNA capping, and tight binding to proteins (analogizing |
The most controversial model, known as '']'', posits that the membrane-bound nucleus, along with other eukaryotic features, originated from the infection of a prokaryote by a virus. The suggestion is based on similarities between eukaryotes and viruses such as linear DNA strands, mRNA capping, and tight binding to proteins (analogizing histones to ]s). One version of the proposal suggests that the nucleus evolved in concert with ] to form an early cellular "]".<ref name="Bell">{{cite journal | vauthors = Bell PJ | title = Viral eukaryogenesis: was the ancestor of the nucleus a complex DNA virus? | journal = Journal of Molecular Evolution | volume = 53 | issue = 3 | pages = 251–6 | date = September 2001 | pmid = 11523012 | doi = 10.1007/s002390010215 | s2cid = 20542871 | department = Comment | bibcode = 2001JMolE..53..251L | doi-access = free }}</ref> Another variant proposes that eukaryotes originated from early archaea infected by ]es, on the basis of observed similarity between the ]s in modern poxviruses and eukaryotes.<ref name="Takemura">{{cite journal | vauthors = Takemura M | title = Poxviruses and the origin of the eukaryotic nucleus | journal = Journal of Molecular Evolution | volume = 52 | issue = 5 | pages = 419–25 | date = May 2001 | pmid = 11443345 | doi = 10.1007/s002390010171 | s2cid = 21200827 | department = Primary | bibcode = 2001JMolE..52..419T }}</ref><ref name="Villareal">{{cite journal | vauthors = Villarreal LP, DeFilippis VR | title = A hypothesis for DNA viruses as the origin of eukaryotic replication proteins | journal = Journal of Virology | volume = 74 | issue = 15 | pages = 7079–84 | date = August 2000 | pmid = 10888648 | pmc = 112226 | doi = 10.1128/JVI.74.15.7079-7084.2000 | department = Primary }}</ref> It has been suggested that the unresolved question of the ] could be related to the viral eukaryogenesis hypothesis.<ref name="Bell2">{{cite journal | vauthors = Bell PJ | title = Sex and the eukaryotic cell cycle is consistent with a viral ancestry for the eukaryotic nucleus | journal = Journal of Theoretical Biology | volume = 243 | issue = 1 | pages = 54–63 | date = November 2006 | pmid = 16846615 | doi = 10.1016/j.jtbi.2006.05.015 | bibcode = 2006JThBi.243...54B | department = Primary <!-- but secondary to the original eukaryogenesis hypothesis --> }}</ref> | ||
| A more recent proposal, the ''exomembrane hypothesis'', suggests that the nucleus instead originated from a single ancestral cell that evolved a second exterior cell membrane; the interior membrane enclosing the original cell then became the nuclear membrane and evolved increasingly elaborate pore structures for passage of internally synthesized cellular components such as |
A more recent proposal, the ''exomembrane hypothesis'', suggests that the nucleus instead originated from a single ancestral cell that evolved a second exterior cell membrane; the interior membrane enclosing the original cell then became the nuclear membrane and evolved increasingly elaborate pore structures for passage of internally synthesized cellular components such as ribosomal subunits.<ref name="deRoos">{{cite journal | vauthors = de Roos AD | title = The origin of the eukaryotic cell based on conservation of existing interfaces | journal = Artificial Life | volume = 12 | issue = 4 | pages = 513–23 | year = 2006 | pmid = 16953783 | doi = 10.1162/artl.2006.12.4.513 | s2cid = 5963228 | department = Primary }}</ref> | ||
| ==History<!--'Cytoblast' redirects here-->== | |||
| ==See also== | |||
| ], 1719]] | |||
| ]'' ] cell published by ] in 1882. The nucleus contains ]s. | |||
| ]] | |||
| The nucleus was the first organelle to be discovered. What is most likely the oldest preserved drawing dates back to the early microscopist Antonie van Leeuwenhoek (1632–1723). He observed a "lumen", the nucleus, in the red blood cells of ].<ref>{{cite book | vauthors = Van Leeuwenhoek A | title = Opera Omnia, seu Arcana Naturae ope exactissimorum Microscopiorum detecta, experimentis variis comprobata, Epistolis ad varios illustres viros J. Arnold et Delphis, A. Beman, Lugdinum Batavorum | trans-title = The Works of, or arcana of nature by means of exactissimorum microscopes had been detected and confirmed by a variety of experiments, the Epistles to the various illustrious men of valor J. Arnold and Delphi, A. Beman, Lugdina York 1719-1730 | language = la }} Cited in {{cite book | vauthors = Gerlach D | title = Geschichte der Mikroskopie | publisher = ] | location = Frankfurt am Main, Germany | date = 2009 | isbn = 978-3-8171-1781-9 }}</ref> Unlike mammalian red blood cells, those of other vertebrates still contain nuclei.<ref>{{cite journal |doi=10.1007/BF01283036 |title=The cytomorphic system of anucleate non-mammalian erythrocytes |year=1982 |vauthors=Cohen WD |journal=Protoplasma |volume=113 |pages=23–32|s2cid=41287948 }}</ref> | |||
| The nucleus was also described by ] in 1804<ref name="Harris">{{cite book | vauthors = Harris H | title =The Birth of the Cell | year =1999 | publisher =Yale University Press | location =New Haven | isbn =978-0-300-07384-3 | url-access =registration | url =https://archive.org/details/birthofcell0000harr }}</ref> and in more detail in 1831 by Scottish ] ] in a talk at the ]. Brown was studying ]s under the microscope when he observed an opaque area, which he called the "areola" or "nucleus", in the cells of the flower's outer layer.<ref name="Robert Brown">{{cite journal | last = Brown | first = Robert | name-list-style = vanc | title = On the Organs and Mode of Fecundation of Orchidex and Asclepiadea | journal = Miscellaneous Botanical Works I | pages = 511–514 | year = 1866}}</ref> He did not suggest a potential function. | |||
| In 1838, ] proposed that the nucleus plays a role in generating cells, thus he introduced the name "'''cytoblast'''<!--boldface per WP:R#PLA-->" ("cell builder"). He believed that he had observed new cells assembling around "cytoblasts". ] was a strong opponent of this view, having already described cells multiplying by division and believing that many cells would have no nuclei. The idea that cells can be generated de novo, by the "cytoblast" or otherwise, contradicted work by ] (1852) and ] (1855) who decisively propagated the new paradigm that cells are generated solely by cells ("{{lang|la|Omnis cellula e cellula}}"). The function of the nucleus remained unclear.<ref name="Cremer">{{cite book | last =Cremer| first =Thomas | name-list-style = vanc | title =Von der Zellenlehre zur Chromosomentheorie | year =1985 | publisher =Springer Verlag | location =Berlin, Heidelberg, New York, Tokyo | isbn = 978-3-540-13987-4}} Online Version </ref> | |||
| Between 1877 and 1878, ] published several studies on the ] of ] eggs, showing that the nucleus of the ] enters the ] and fuses with its nucleus. This was the first time it was suggested that an individual develops from a (single) nucleated cell. This was in contradiction to ]'s theory that the complete ] of a species would be repeated during embryonic development, including generation of the first nucleated cell from a "monerula", a structureless mass of primordial protoplasm ("]"). Therefore, the necessity of the sperm nucleus for fertilization was discussed for quite some time. However, Hertwig confirmed his observation in other animal groups, including ] and ]. ] produced the same results for plants in 1884. This paved the way to assign the nucleus an important role in heredity. In 1873, ] postulated the equivalence of the maternal and paternal germ ''cells'' for heredity. The function of the nucleus as carrier of genetic information became clear only later, after mitosis was discovered and the ] were rediscovered at the beginning of the 20th century; the ] was therefore developed.<ref name ="Cremer"/> | |||
| == See also == | |||
| * ] | |||
| * ] | * ] | ||
| * ] | |||
| * ] | |||
| == |
== References == | ||
| {{Reflist}} | |||
| <gallery class="center" > | |||
| Image:Chr2 orang human.jpg|Comparison of human and ] chromosomes. | |||
| Image:MouseChromosomeTerritoriesBMC Cell Biol6-44Fig2.jpg|Mouse chromosome territories in different cell types. | |||
| Image:PLoSBiol3.5.Fig1bNucleus46Chromosomes.jpg|24 chromosome territories in human cells. | |||
| </gallery> | |||
| == Further reading == | |||
| ==References== | |||
| {{ |
{{refbegin|30em}} | ||
| * {{cite journal | vauthors = Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP | title = Nuclear lamins: building blocks of nuclear architecture | journal = Genes & Development | volume = 16 | issue = 5 | pages = 533–47 | date = March 2002 | pmid = 11877373 | doi = 10.1101/gad.960502 | doi-access = free }} | |||
| :A review article about nuclear lamins, explaining their structure and various roles | |||
| * {{cite journal | vauthors = Görlich D, Kutay U | title = Transport between the cell nucleus and the cytoplasm | journal = Annual Review of Cell and Developmental Biology | volume = 15 | pages = 607–60 | year = 1999 | pmid = 10611974 | doi = 10.1146/annurev.cellbio.15.1.607 }} | |||
| :A review article about nuclear transport, explains the principles of the mechanism, and the various transport pathways | |||
| * {{cite journal | vauthors = Lamond AI, Earnshaw WC | title = Structure and function in the nucleus | journal = Science | volume = 280 | issue = 5363 | pages = 547–53 | date = April 1998 | pmid = 9554838 | doi = 10.1126/science.280.5363.547 | url = http://azolla.fc.ul.pt/aulas/BiologiaCelular/docs/nucleo.pdf | citeseerx = 10.1.1.323.5543 }} | |||
| :A review article about the nucleus, explaining the structure of chromosomes within the organelle, and describing the nucleolus and other subnuclear bodies | |||
| * {{cite journal | vauthors = Pennisi E | title = Evolutionary biology. The birth of the nucleus | journal = Science | volume = 305 | issue = 5685 | pages = 766–8 | date = August 2004 | pmid = 15297641 | doi = 10.1126/science.305.5685.766 | s2cid = 83769250 }} | |||
| :A review article about the evolution of the nucleus, explaining a number of different theories | |||
| * {{cite book | vauthors = Pollard TD, Earnshaw WC | title = Cell Biology | publisher = Saunders | year = 2004 | location = Philadelphia | isbn = 978-0-7216-3360-2 }} | |||
| :A university level textbook focusing on cell biology. Contains information on nucleus structure and function, including nuclear transport, and subnuclear domains | |||
| {{refend}} | |||
| == External links == | |||
| ==Further reading== | |||
| {{Commons category|Cell nucleus}} | |||
| {{refbegin}} | |||
| {{Library resources box | {{Library resources box | ||
| |onlinebooks=yes | |onlinebooks=yes | ||
| Line 327: | Line 262: | ||
| |label=Cell nucleus | |label=Cell nucleus | ||
| }} | }} | ||
| * {{cite web | title = The Nucleus | url = http://www.mechanobio.info/topics/cellular-organization/go-0005634 | work = MBInfo }} | |||
| * {{cite journal | |||
| * {{cite web | title = Learn about the Cell Nucleus | work = cellnucleus.com | url = http://www.cellnucleus.com/education_main.htm }} Website covering structure and function of the nucleus from the Department of Oncology at the University of Alberta. | |||
| | last = Goldman | |||
| * {{cite web | vauthors = Bickmore W | url = http://npd.hgu.mrc.ac.uk/user/?page=compartment | title = The Nuclear Protein Database | publisher = Medical Research Council Human Genetics Unit }} Information on nuclear components. | |||
| | first = Robert D. | |||
| * {{cite web | title = The Nucleus Collection | url = http://cellimages.ascb.org/cdm4/browse.php?CISOROOT=%2Fp4041coll6 | work = Image & Video Library | publisher = The American Society for Cell Biology | archive-url = https://web.archive.org/web/20061112023405/http://cellimages.ascb.org/cdm4/browse.php?CISOROOT=%2Fp4041coll6 | archive-date = 12 November 2006 }} contains peer-reviewed still images and video clips that illustrate the nucleus. | |||
| | title = Nuclear lamins: building blocks of nuclear architecture | |||
| * {{cite web | title = Nuclear Envelope and Nuclear Import Section | url = http://cellimages.ascb.org/u/?%2Fp4041coll11%2C62 | work = Landmark Papers in Cell Biology | archive-url = https://web.archive.org/web/20061117175431/http://cellimages.ascb.org/u/?%2Fp4041coll11%2C62 | archive-date = 17 November 2006 | veditors = Gall JG, McIntosh JR }} contains digitized commentaries and links to seminal research papers on the nucleus. Published online in the {{Webarchive|url=https://web.archive.org/web/20110610012208/http://cellimages.ascb.org/ |date=10 June 2011 }} of | |||
| | journal = Genes & Dev. | |||
| * {{cite web | title = Cytoplasmic patterns generated by human antibodies | url = http://www.antibodypatterns.com/cytoplasmic.php | work = AntibodyPatterns.com | archive-url = https://web.archive.org/web/20070102032704/http://www.antibodypatterns.com/cytoplasmic.php | archive-date = 2 January 2007 }} | |||
| | issue = 5 | |||
| {{refend}} | |||
| | pages = 533–547 | |||
| | year = 2002 | |||
| | doi = 10.1101/gad.960502 | |||
| | volume = 16 | |||
| | pmid = 11877373 | |||
| | last2 = Gruenbaum | |||
| | first2 = Y | |||
| | last3 = Moir | |||
| | first3 = RD | |||
| | last4 = Shumaker | |||
| | first4 = DK | |||
| | last5 = Spann | |||
| | first5 = TP | |||
| }} | |||
| :A review article about nuclear lamins, explaining their structure and various roles | |||
| * {{cite journal | last = Görlich | first = Dirk | title = Transport between the cell nucleus and the cytoplasm | journal = Ann. Rev. Cell Dev. Biol. | volume = 15| issue = | pages = 607–660 | year = 1999 | pmid =10611974 | doi = 10.1146/annurev.cellbio.15.1.607 | last2 = Kutay | first2 = U }} | |||
| :A review article about nuclear transport, explains the principles of the mechanism, and the various transport pathways | |||
| * {{cite journal | last = Lamond | first = Angus I. | title = Structure and Function in the Nucleus | journal = Science | volume = 280 | pages = 547–553 | date = 1998-04-24 | pmid =9554838 | doi = 10.1126/science.280.5363.547 | last2 = Earnshaw | first2 = WC | issue = 5363}} | |||
| :A review article about the nucleus, explaining the structure of chromosomes within the organelle, and describing the nucleolus and other subnuclear bodies | |||
| * {{cite journal | author = Pennisi E.| title = Evolutionary biology. The birth of the nucleus | doi = 10.1126/science.305.5685.766| journal = Science | volume = 305 | issue =5685 | pages = 766–768 | year =2004 | pmid = 15297641}} | |||
| :A review article about the evolution of the nucleus, explaining a number of different theories | |||
| * {{cite book | |||
| | last = Pollard | |||
| | first = Thomas D. | |||
| |author2=William C. Earnshaw | |||
| | title = Cell Biology | |||
| | publisher = Saunders | |||
| | year = 2004 | |||
| | location = Philadelphia | |||
| | isbn = 0-7216-3360-9}} | |||
| :A university level textbook focusing on cell biology. Contains information on nucleus structure and function, including nuclear transport, and subnuclear domains | |||
| ==External links== | |||
| {{Commons category|Cell nucleus}} | |||
| * | |||
| * Website covering structure and function of the nucleus from the Department of Oncology at the University of Alberta. | |||
| * http://npd.hgu.mrc.ac.uk/user/?page=compartment The Nuclear Protein Database] Information on nuclear components. | |||
| * in the of contains peer-reviewed still images and video clips that illustrate the nucleus. | |||
| * from ,'' Joseph G. Gall, J. Richard McIntosh, eds., contains digitized commentaries and links to seminal research papers on the nucleus. Published online in the of | |||
| * | |||
| {{organelles}} | {{organelles}} | ||
| {{Nucleus}} | {{Nucleus}} | ||
| {{featured article}} | |||
| {{Authority control}} | {{Authority control}} | ||
| {{DEFAULTSORT:Cell Nucleus}} | {{DEFAULTSORT:Cell Nucleus}} | ||
| ] | ] | ||
Latest revision as of 18:00, 4 December 2024
Eukaryotic membrane-bounded organelle containing DNA

| Cell biology | |
|---|---|
| Animal cell diagram | |
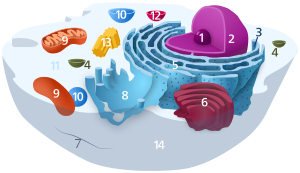 Components of a typical animal cell: Components of a typical animal cell:
|
The cell nucleus (from Latin nucleus or nuculeus 'kernel, seed'; pl.: nuclei) is a membrane-bound organelle found in eukaryotic cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, have no nuclei, and a few others including osteoclasts have many. The main structures making up the nucleus are the nuclear envelope, a double membrane that encloses the entire organelle and isolates its contents from the cellular cytoplasm; and the nuclear matrix, a network within the nucleus that adds mechanical support.
The cell nucleus contains nearly all of the cell's genome. Nuclear DNA is often organized into multiple chromosomes – long strands of DNA dotted with various proteins, such as histones, that protect and organize the DNA. The genes within these chromosomes are structured in such a way to promote cell function. The nucleus maintains the integrity of genes and controls the activities of the cell by regulating gene expression.
Because the nuclear envelope is impermeable to large molecules, nuclear pores are required to regulate nuclear transport of molecules across the envelope. The pores cross both nuclear membranes, providing a channel through which larger molecules must be actively transported by carrier proteins while allowing free movement of small molecules and ions. Movement of large molecules such as proteins and RNA through the pores is required for both gene expression and the maintenance of chromosomes. Although the interior of the nucleus does not contain any membrane-bound subcompartments, a number of nuclear bodies exist, made up of unique proteins, RNA molecules, and particular parts of the chromosomes. The best-known of these is the nucleolus, involved in the assembly of ribosomes.
Chromosomes
Main article: Chromosome Further information: Nuclear organization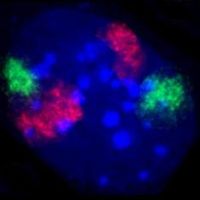
The cell nucleus contains the majority of the cell's genetic material in the form of multiple linear DNA molecules organized into structures called chromosomes. Each human cell contains roughly two meters of DNA. During most of the cell cycle these are organized in a DNA-protein complex known as chromatin, and during cell division the chromatin can be seen to form the well-defined chromosomes familiar from a karyotype. A small fraction of the cell's genes are located instead in the mitochondria.
There are two types of chromatin. Euchromatin is the less compact DNA form, and contains genes that are frequently expressed by the cell. The other type, heterochromatin, is the more compact form, and contains DNA that is infrequently transcribed. This structure is further categorized into facultative heterochromatin, consisting of genes that are organized as heterochromatin only in certain cell types or at certain stages of development, and constitutive heterochromatin that consists of chromosome structural components such as telomeres and centromeres. During interphase the chromatin organizes itself into discrete individual patches, called chromosome territories. Active genes, which are generally found in the euchromatic region of the chromosome, tend to be located towards the chromosome's territory boundary.
Antibodies to certain types of chromatin organization, in particular, nucleosomes, have been associated with a number of autoimmune diseases, such as systemic lupus erythematosus. These are known as anti-nuclear antibodies (ANA) and have also been observed in concert with multiple sclerosis as part of general immune system dysfunction.
Nuclear structures and landmarks
Further information: Nuclear equivalence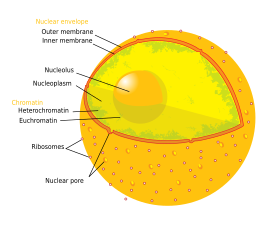
The nucleus contains nearly all of the cell's DNA, surrounded by a network of fibrous intermediate filaments called the nuclear matrix, and is enveloped in a double membrane called the nuclear envelope. The nuclear envelope separates the fluid inside the nucleus, called the nucleoplasm, from the rest of the cell. The size of the nucleus is correlated to the size of the cell, and this ratio is reported across a range of cell types and species. In eukaryotes the nucleus in many cells typically occupies 10% of the cell volume. The nucleus is the largest organelle in animal cells. In human cells, the diameter of the nucleus is approximately six micrometres (μm).
Nuclear envelope and pores
Main articles: Nuclear envelope and Nuclear pore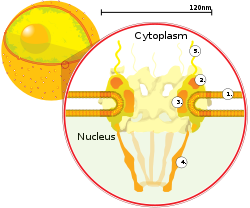
The nuclear envelope consists of two membranes, an inner and an outer nuclear membrane, perforated by nuclear pores. Together, these membranes serve to separate the cell's genetic material from the rest of the cell contents, and allow the nucleus to maintain an environment distinct from the rest of the cell. Despite their close apposition around much of the nucleus, the two membranes differ substantially in shape and contents. The inner membrane surrounds the nuclear content, providing its defining edge. Embedded within the inner membrane, various proteins bind the intermediate filaments that give the nucleus its structure. The outer membrane encloses the inner membrane, and is continuous with the adjacent endoplasmic reticulum membrane. As part of the endoplasmic reticulum membrane, the outer nuclear membrane is studded with ribosomes that are actively translating proteins across membrane. The space between the two membranes is called the perinuclear space, and is continuous with the endoplasmic reticulum lumen.
In a mammalian nuclear envelope there are between 3000 and 4000 nuclear pore complexes (NPCs) perforating the envelope. Each NPC contains an eightfold-symmetric ring-shaped structure at a position where the inner and outer membranes fuse. The number of NPCs can vary considerably across cell types; small glial cells only have about a few hundred, with large Purkinje cells having around 20,000. The NPC provides selective transport of molecules between the nucleoplasm and the cytosol. The nuclear pore complex is composed of approximately thirty different proteins known as nucleoporins. The pores are about 60–80 million daltons in molecular weight and consist of around 50 (in yeast) to several hundred proteins (in vertebrates). The pores are 100 nm in total diameter; however, the gap through which molecules freely diffuse is only about 9 nm wide, due to the presence of regulatory systems within the center of the pore. This size selectively allows the passage of small water-soluble molecules while preventing larger molecules, such as nucleic acids and larger proteins, from inappropriately entering or exiting the nucleus. These large molecules must be actively transported into the nucleus instead. Attached to the ring is a structure called the nuclear basket that extends into the nucleoplasm, and a series of filamentous extensions that reach into the cytoplasm. Both structures serve to mediate binding to nuclear transport proteins.
Most proteins, ribosomal subunits, and some RNAs are transported through the pore complexes in a process mediated by a family of transport factors known as karyopherins. Those karyopherins that mediate movement into the nucleus are also called importins, whereas those that mediate movement out of the nucleus are called exportins. Most karyopherins interact directly with their cargo, although some use adaptor proteins. Steroid hormones such as cortisol and aldosterone, as well as other small lipid-soluble molecules involved in intercellular signaling, can diffuse through the cell membrane and into the cytoplasm, where they bind nuclear receptor proteins that are trafficked into the nucleus. There they serve as transcription factors when bound to their ligand; in the absence of a ligand, many such receptors function as histone deacetylases that repress gene expression.
Nuclear lamina
Main article: Nuclear laminaIn animal cells, two networks of intermediate filaments provide the nucleus with mechanical support: The nuclear lamina forms an organized meshwork on the internal face of the envelope, while less organized support is provided on the cytosolic face of the envelope. Both systems provide structural support for the nuclear envelope and anchoring sites for chromosomes and nuclear pores.
The nuclear lamina is composed mostly of lamin proteins. Like all proteins, lamins are synthesized in the cytoplasm and later transported to the nucleus interior, where they are assembled before being incorporated into the existing network of nuclear lamina. Lamins found on the cytosolic face of the membrane, such as emerin and nesprin, bind to the cytoskeleton to provide structural support. Lamins are also found inside the nucleoplasm where they form another regular structure, known as the nucleoplasmic veil, that is visible using fluorescence microscopy. The actual function of the veil is not clear, although it is excluded from the nucleolus and is present during interphase. Lamin structures that make up the veil, such as LEM3, bind chromatin and disrupting their structure inhibits transcription of protein-coding genes.
Like the components of other intermediate filaments, the lamin monomer contains an alpha-helical domain used by two monomers to coil around each other, forming a dimer structure called a coiled coil. Two of these dimer structures then join side by side, in an antiparallel arrangement, to form a tetramer called a protofilament. Eight of these protofilaments form a lateral arrangement that is twisted to form a ropelike filament. These filaments can be assembled or disassembled in a dynamic manner, meaning that changes in the length of the filament depend on the competing rates of filament addition and removal.
Mutations in lamin genes leading to defects in filament assembly cause a group of rare genetic disorders known as laminopathies. The most notable laminopathy is the family of diseases known as progeria, which causes the appearance of premature aging in those with the condition. The exact mechanism by which the associated biochemical changes give rise to the aged phenotype is not well understood.
Nucleolus
Main article: Nucleolus Further information: Nuclear bodies
The nucleolus is the largest of the discrete densely stained, membraneless structures known as nuclear bodies found in the nucleus. It forms around tandem repeats of rDNA, DNA coding for ribosomal RNA (rRNA). These regions are called nucleolar organizer regions (NOR). The main roles of the nucleolus are to synthesize rRNA and assemble ribosomes. The structural cohesion of the nucleolus depends on its activity, as ribosomal assembly in the nucleolus results in the transient association of nucleolar components, facilitating further ribosomal assembly, and hence further association. This model is supported by observations that inactivation of rDNA results in intermingling of nucleolar structures.
In the first step of ribosome assembly, a protein called RNA polymerase I transcribes rDNA, which forms a large pre-rRNA precursor. This is cleaved into two large rRNA subunits – 5.8S, and 28S, and a small rRNA subunit 18S. The transcription, post-transcriptional processing, and assembly of rRNA occurs in the nucleolus, aided by small nucleolar RNA (snoRNA) molecules, some of which are derived from spliced introns from messenger RNAs encoding genes related to ribosomal function. The assembled ribosomal subunits are the largest structures passed through the nuclear pores.
When observed under the electron microscope, the nucleolus can be seen to consist of three distinguishable regions: the innermost fibrillar centers (FCs), surrounded by the dense fibrillar component (DFC) (that contains fibrillarin and nucleolin), which in turn is bordered by the granular component (GC) (that contains the protein nucleophosmin). Transcription of the rDNA occurs either in the FC or at the FC-DFC boundary, and, therefore, when rDNA transcription in the cell is increased, more FCs are detected. Most of the cleavage and modification of rRNAs occurs in the DFC, while the latter steps involving protein assembly onto the ribosomal subunits occur in the GC.
Splicing speckles
Speckles are subnuclear structures that are enriched in pre-messenger RNA splicing factors and are located in the interchromatin regions of the nucleoplasm of mammalian cells. At the fluorescence-microscope level they appear as irregular, punctate structures, which vary in size and shape, and when examined by electron microscopy they are seen as clusters of interchromatin granules. Speckles are dynamic structures, and both their protein and RNA-protein components can cycle continuously between speckles and other nuclear locations, including active transcription sites. Speckles can work with p53 as enhancers of gene activity to directly enhance the activity of certain genes. Moreover, speckle-associating and non-associating p53 gene targets are functionally distinct.
Studies on the composition, structure and behaviour of speckles have provided a model for understanding the functional compartmentalization of the nucleus and the organization of the gene-expression machinery splicing snRNPs and other splicing proteins necessary for pre-mRNA processing. Because of a cell's changing requirements, the composition and location of these bodies changes according to mRNA transcription and regulation via phosphorylation of specific proteins. The splicing speckles are also known as nuclear speckles (nuclear specks), splicing factor compartments (SF compartments), interchromatin granule clusters (IGCs), and B snurposomes. B snurposomes are found in the amphibian oocyte nuclei and in Drosophila melanogaster embryos. B snurposomes appear alone or attached to the Cajal bodies in the electron micrographs of the amphibian nuclei. While nuclear speckles were originally thought to be storage sites for the splicing factors, a more recent study demonstrated that organizing genes and pre-mRNA substrates near speckles increases the kinetic efficiency of pre-mRNA splicing, ultimately boosting protein levels by modulation of splicing.
Cajal bodies and gems

A nucleus typically contains between one and ten compact structures called Cajal bodies or coiled bodies (CB), whose diameter measures between 0.2 μm and 2.0 μm depending on the cell type and species. When seen under an electron microscope, they resemble balls of tangled thread and are dense foci of distribution for the protein coilin. CBs are involved in a number of different roles relating to RNA processing, specifically small nucleolar RNA (snoRNA) and small nuclear RNA (snRNA) maturation, and histone mRNA modification.
Similar to Cajal bodies are Gemini of Cajal bodies, or gems, whose name is derived from the Gemini constellation in reference to their close "twin" relationship with CBs. Gems are similar in size and shape to CBs, and in fact are virtually indistinguishable under the microscope. Unlike CBs, gems do not contain small nuclear ribonucleoproteins (snRNPs), but do contain a protein called survival of motor neuron (SMN) whose function relates to snRNP biogenesis. Gems are believed to assist CBs in snRNP biogenesis, though it has also been suggested from microscopy evidence that CBs and gems are different manifestations of the same structure. Later ultrastructural studies have shown gems to be twins of Cajal bodies with the difference being in the coilin component; Cajal bodies are SMN positive and coilin positive, and gems are SMN positive and coilin negative.
Other nuclear bodies
Main article: Nuclear bodies| Structure name | Structure diameter | Ref. |
|---|---|---|
| Cajal bodies | 0.2–2.0 μm | |
| Clastosomes | 0.2–0.5 μm | |
| PIKA | 5 μm | |
| PML bodies | 0.2–1.0 μm | |
| Paraspeckles | 0.5–1.0 μm | |
| Speckles | 20–25 nm |
Beyond the nuclear bodies first described by Santiago Ramón y Cajal above (e.g., nucleolus, nuclear speckles, Cajal bodies) the nucleus contains a number of other nuclear bodies. These include polymorphic interphase karyosomal association (PIKA), promyelocytic leukaemia (PML) bodies, and paraspeckles. Although little is known about a number of these domains, they are significant in that they show that the nucleoplasm is not a uniform mixture, but rather contains organized functional subdomains.
Other subnuclear structures appear as part of abnormal disease processes. For example, the presence of small intranuclear rods has been reported in some cases of nemaline myopathy. This condition typically results from mutations in actin, and the rods themselves consist of mutant actin as well as other cytoskeletal proteins.
PIKA and PTF domains
PIKA domains, or polymorphic interphase karyosomal associations, were first described in microscopy studies in 1991. Their function remains unclear, though they were not thought to be associated with active DNA replication, transcription, or RNA processing. They have been found to often associate with discrete domains defined by dense localization of the transcription factor PTF, which promotes transcription of small nuclear RNA (snRNA).
PML-nuclear bodies
Promyelocytic leukemia protein (PML-nuclear bodies) are spherical bodies found scattered throughout the nucleoplasm, measuring around 0.1–1.0 μm. They are known by a number of other names, including nuclear domain 10 (ND10), Kremer bodies, and PML oncogenic domains. PML-nuclear bodies are named after one of their major components, the promyelocytic leukemia protein (PML). They are often seen in the nucleus in association with Cajal bodies and cleavage bodies. Pml-/- mice, which are unable to create PML-nuclear bodies, develop normally without obvious ill effects, showing that PML-nuclear bodies are not required for most essential biological processes.
Paraspeckles
Main article: ParaspeckleDiscovered by Fox et al. in 2002, paraspeckles are irregularly shaped compartments in the interchromatin space of the nucleus. First documented in HeLa cells, where there are generally 10–30 per nucleus, paraspeckles are now known to also exist in all human primary cells, transformed cell lines, and tissue sections. Their name is derived from their distribution in the nucleus; the "para" is short for parallel and the "speckles" refers to the splicing speckles to which they are always in close proximity.
Paraspeckles sequester nuclear proteins and RNA and thus appear to function as a molecular sponge that is involved in the regulation of gene expression. Furthermore, paraspeckles are dynamic structures that are altered in response to changes in cellular metabolic activity. They are transcription dependent and in the absence of RNA Pol II transcription, the paraspeckle disappears and all of its associated protein components (PSP1, p54nrb, PSP2, CFI(m)68, and PSF) form a crescent shaped perinucleolar cap in the nucleolus. This phenomenon is demonstrated during the cell cycle. In the cell cycle, paraspeckles are present during interphase and during all of mitosis except for telophase. During telophase, when the two daughter nuclei are formed, there is no RNA Pol II transcription so the protein components instead form a perinucleolar cap.
Perichromatin fibrils
Perichromatin fibrils are visible only under electron microscope. They are located next to the transcriptionally active chromatin and are hypothesized to be the sites of active pre-mRNA processing.
Clastosomes
Clastosomes are small nuclear bodies (0.2–0.5 μm) described as having a thick ring-shape due to the peripheral capsule around these bodies. This name is derived from the Greek klastos (κλαστός), broken and soma (σῶμα), body. Clastosomes are not typically present in normal cells, making them hard to detect. They form under high proteolytic conditions within the nucleus and degrade once there is a decrease in activity or if cells are treated with proteasome inhibitors. The scarcity of clastosomes in cells indicates that they are not required for proteasome function. Osmotic stress has also been shown to cause the formation of clastosomes. These nuclear bodies contain catalytic and regulatory subunits of the proteasome and its substrates, indicating that clastosomes are sites for degrading proteins.
Function
The nucleus provides a site for genetic transcription that is segregated from the location of translation in the cytoplasm, allowing levels of gene regulation that are not available to prokaryotes. The main function of the cell nucleus is to control gene expression and mediate the replication of DNA during the cell cycle.
Cell compartmentalization
The nuclear envelope allows control of the nuclear contents, and separates them from the rest of the cytoplasm where necessary. This is important for controlling processes on either side of the nuclear membrane: In most cases where a cytoplasmic process needs to be restricted, a key participant is removed to the nucleus, where it interacts with transcription factors to downregulate the production of certain enzymes in the pathway. This regulatory mechanism occurs in the case of glycolysis, a cellular pathway for breaking down glucose to produce energy. Hexokinase is an enzyme responsible for the first step of glycolysis, forming glucose-6-phosphate from glucose. At high concentrations of fructose-6-phosphate, a molecule made later from glucose-6-phosphate, a regulator protein removes hexokinase to the nucleus, where it forms a transcriptional repressor complex with nuclear proteins to reduce the expression of genes involved in glycolysis.
In order to control which genes are being transcribed, the cell separates some transcription factor proteins responsible for regulating gene expression from physical access to the DNA until they are activated by other signaling pathways. This prevents even low levels of inappropriate gene expression. For example, in the case of NF-κB-controlled genes, which are involved in most inflammatory responses, transcription is induced in response to a signal pathway such as that initiated by the signaling molecule TNF-α, binds to a cell membrane receptor, resulting in the recruitment of signalling proteins, and eventually activating the transcription factor NF-κB. A nuclear localisation signal on the NF-κB protein allows it to be transported through the nuclear pore and into the nucleus, where it stimulates the transcription of the target genes.
The compartmentalization allows the cell to prevent translation of unspliced mRNA. Eukaryotic mRNA contains introns that must be removed before being translated to produce functional proteins. The splicing is done inside the nucleus before the mRNA can be accessed by ribosomes for translation. Without the nucleus, ribosomes would translate newly transcribed (unprocessed) mRNA, resulting in malformed and nonfunctional proteins.
Replication
Main article: Eukaryotic DNA replicationThe main function of the cell nucleus is to control gene expression and mediate the replication of DNA during the cell cycle. It has been found that replication happens in a localised way in the cell nucleus. In the S phase of interphase of the cell cycle; replication takes place. Contrary to the traditional view of moving replication forks along stagnant DNA, a concept of replication factories emerged, which means replication forks are concentrated towards some immobilised 'factory' regions through which the template DNA strands pass like conveyor belts.
Gene expression
Main article: Gene expression See also: Transcription factories
Gene expression first involves transcription, in which DNA is used as a template to produce RNA. In the case of genes encoding proteins, that RNA produced from this process is messenger RNA (mRNA), which then needs to be translated by ribosomes to form a protein. As ribosomes are located outside the nucleus, mRNA produced needs to be exported.
Since the nucleus is the site of transcription, it also contains a variety of proteins that either directly mediate transcription or are involved in regulating the process. These proteins include helicases, which unwind the double-stranded DNA molecule to facilitate access to it, RNA polymerases, which bind to the DNA promoter to synthesize the growing RNA molecule, topoisomerases, which change the amount of supercoiling in DNA, helping it wind and unwind, as well as a large variety of transcription factors that regulate expression.
Processing of pre-mRNA
Main article: Post-transcriptional modificationNewly synthesized mRNA molecules are known as primary transcripts or pre-mRNA. They must undergo post-transcriptional modification in the nucleus before being exported to the cytoplasm; mRNA that appears in the cytoplasm without these modifications is degraded rather than used for protein translation. The three main modifications are 5' capping, 3' polyadenylation, and RNA splicing. While in the nucleus, pre-mRNA is associated with a variety of proteins in complexes known as heterogeneous ribonucleoprotein particles (hnRNPs). Addition of the 5' cap occurs co-transcriptionally and is the first step in post-transcriptional modification. The 3' poly-adenine tail is only added after transcription is complete.
RNA splicing, carried out by a complex called the spliceosome, is the process by which introns, or regions of DNA that do not code for protein, are removed from the pre-mRNA and the remaining exons connected to re-form a single continuous molecule. This process normally occurs after 5' capping and 3' polyadenylation but can begin before synthesis is complete in transcripts with many exons. Many pre-mRNAs can be spliced in multiple ways to produce different mature mRNAs that encode different protein sequences. This process is known as alternative splicing, and allows production of a large variety of proteins from a limited amount of DNA.
Dynamics and regulation
Nuclear transport
Main article: Nuclear transport
The entry and exit of large molecules from the nucleus is tightly controlled by the nuclear pore complexes. Although small molecules can enter the nucleus without regulation, macromolecules such as RNA and proteins require association karyopherins called importins to enter the nucleus and exportins to exit. "Cargo" proteins that must be translocated from the cytoplasm to the nucleus contain short amino acid sequences known as nuclear localization signals, which are bound by importins, while those transported from the nucleus to the cytoplasm carry nuclear export signals bound by exportins. The ability of importins and exportins to transport their cargo is regulated by GTPases, enzymes that hydrolyze the molecule guanosine triphosphate (GTP) to release energy. The key GTPase in nuclear transport is Ran, which is bound to either GTP or GDP (guanosine diphosphate), depending on whether it is located in the nucleus or the cytoplasm. Whereas importins depend on RanGTP to dissociate from their cargo, exportins require RanGTP in order to bind to their cargo.
Nuclear import depends on the importin binding its cargo in the cytoplasm and carrying it through the nuclear pore into the nucleus. Inside the nucleus, RanGTP acts to separate the cargo from the importin, allowing the importin to exit the nucleus and be reused. Nuclear export is similar, as the exportin binds the cargo inside the nucleus in a process facilitated by RanGTP, exits through the nuclear pore, and separates from its cargo in the cytoplasm.
Specialized export proteins exist for translocation of mature mRNA and tRNA to the cytoplasm after post-transcriptional modification is complete. This quality-control mechanism is important due to these molecules' central role in protein translation. Mis-expression of a protein due to incomplete excision of exons or mis-incorporation of amino acids could have negative consequences for the cell; thus, incompletely modified RNA that reaches the cytoplasm is degraded rather than used in translation.
Assembly and disassembly

During its lifetime, a nucleus may be broken down or destroyed, either in the process of cell division or as a consequence of apoptosis (the process of programmed cell death). During these events, the structural components of the nucleus — the envelope and lamina — can be systematically degraded. In most cells, the disassembly of the nuclear envelope marks the end of the prophase of mitosis. However, this disassembly of the nucleus is not a universal feature of mitosis and does not occur in all cells. Some unicellular eukaryotes (e.g., yeasts) undergo so-called closed mitosis, in which the nuclear envelope remains intact. In closed mitosis, the daughter chromosomes migrate to opposite poles of the nucleus, which then divides in two. The cells of higher eukaryotes, however, usually undergo open mitosis, which is characterized by breakdown of the nuclear envelope. The daughter chromosomes then migrate to opposite poles of the mitotic spindle, and new nuclei reassemble around them.
At a certain point during the cell cycle in open mitosis, the cell divides to form two cells. In order for this process to be possible, each of the new daughter cells must have a full set of genes, a process requiring replication of the chromosomes as well as segregation of the separate sets. This occurs by the replicated chromosomes, the sister chromatids, attaching to microtubules, which in turn are attached to different centrosomes. The sister chromatids can then be pulled to separate locations in the cell. In many cells, the centrosome is located in the cytoplasm, outside the nucleus; the microtubules would be unable to attach to the chromatids in the presence of the nuclear envelope. Therefore, the early stages in the cell cycle, beginning in prophase and until around prometaphase, the nuclear membrane is dismantled. Likewise, during the same period, the nuclear lamina is also disassembled, a process regulated by phosphorylation of the lamins by protein kinases such as the CDC2 protein kinase. Towards the end of the cell cycle, the nuclear membrane is reformed, and around the same time, the nuclear lamina are reassembled by dephosphorylating the lamins.
However, in dinoflagellates, the nuclear envelope remains intact, the centrosomes are located in the cytoplasm, and the microtubules come in contact with chromosomes, whose centromeric regions are incorporated into the nuclear envelope (the so-called closed mitosis with extranuclear spindle). In many other protists (e.g., ciliates, sporozoans) and fungi, the centrosomes are intranuclear, and their nuclear envelope also does not disassemble during cell division.
Apoptosis is a controlled process in which the cell's structural components are destroyed, resulting in death of the cell. Changes associated with apoptosis directly affect the nucleus and its contents, for example, in the condensation of chromatin and the disintegration of the nuclear envelope and lamina. The destruction of the lamin networks is controlled by specialized apoptotic proteases called caspases, which cleave the lamin proteins and, thus, degrade the nucleus' structural integrity. Lamin cleavage is sometimes used as a laboratory indicator of caspase activity in assays for early apoptotic activity. Cells that express mutant caspase-resistant lamins are deficient in nuclear changes related to apoptosis, suggesting that lamins play a role in initiating the events that lead to apoptotic degradation of the nucleus. Inhibition of lamin assembly itself is an inducer of apoptosis.
The nuclear envelope acts as a barrier that prevents both DNA and RNA viruses from entering the nucleus. Some viruses require access to proteins inside the nucleus in order to replicate and/or assemble. DNA viruses, such as herpesvirus replicate and assemble in the cell nucleus, and exit by budding through the inner nuclear membrane. This process is accompanied by disassembly of the lamina on the nuclear face of the inner membrane.
Disease-related dynamics
Initially, it has been suspected that immunoglobulins in general and autoantibodies in particular do not enter the nucleus. Now there is a body of evidence that under pathological conditions (e.g. lupus erythematosus) IgG can enter the nucleus.
Nuclei per cell
Most eukaryotic cell types usually have a single nucleus, but some have no nuclei, while others have several. This can result from normal development, as in the maturation of mammalian red blood cells, or from faulty cell division.
Anucleated cells
An anucleated cell contains no nucleus and is, therefore, incapable of dividing to produce daughter cells. The best-known anucleated cell is the mammalian red blood cell, or erythrocyte, which also lacks other organelles such as mitochondria, and serves primarily as a transport vessel to ferry oxygen from the lungs to the body's tissues. Erythrocytes mature through erythropoiesis in the bone marrow, where they lose their nuclei, organelles, and ribosomes. The nucleus is expelled during the process of differentiation from an erythroblast to a reticulocyte, which is the immediate precursor of the mature erythrocyte. The presence of mutagens may induce the release of some immature "micronucleated" erythrocytes into the bloodstream. Anucleated cells can also arise from flawed cell division in which one daughter lacks a nucleus and the other has two nuclei.
In flowering plants, this condition occurs in sieve tube elements.
Multinucleated cells
Main article: MultinucleateMultinucleated cells contain multiple nuclei. Most acantharean species of protozoa and some fungi in mycorrhizae have naturally multinucleated cells. Other examples include the intestinal parasites in the genus Giardia, which have two nuclei per cell. Ciliates have two kinds of nuclei in a single cell, a somatic macronucleus and a germline micronucleus. In humans, skeletal muscle cells, also called myocytes and syncytium, become multinucleated during development; the resulting arrangement of nuclei near the periphery of the cells allows maximal intracellular space for myofibrils. Other multinucleate cells in the human are osteoclasts a type of bone cell. Multinucleated and binucleated cells can also be abnormal in humans; for example, cells arising from the fusion of monocytes and macrophages, known as giant multinucleated cells, sometimes accompany inflammation and are also implicated in tumor formation.
A number of dinoflagellates are known to have two nuclei. Unlike other multinucleated cells these nuclei contain two distinct lineages of DNA: one from the dinoflagellate and the other from a symbiotic diatom.
Evolution
As the major defining characteristic of the eukaryotic cell, the nucleus's evolutionary origin has been the subject of much speculation. Four major hypotheses have been proposed to explain the existence of the nucleus, although none have yet earned widespread support.
The first model known as the "syntrophic model" proposes that a symbiotic relationship between the archaea and bacteria created the nucleus-containing eukaryotic cell. (Organisms of the Archaeal and Bacterial domains have no cell nucleus.) It is hypothesized that the symbiosis originated when ancient archaea similar to modern methanogenic archaea, invaded and lived within bacteria similar to modern myxobacteria, eventually forming the early nucleus. This theory is analogous to the accepted theory for the origin of eukaryotic mitochondria and chloroplasts, which are thought to have developed from a similar endosymbiotic relationship between proto-eukaryotes and aerobic bacteria. One possibility is that the nuclear membrane arose as a new membrane system following the origin of mitochondria in an archaebacterial host. The nuclear membrane may have served to protect the genome from damaging reactive oxygen species produced by the protomitochondria. The archaeal origin of the nucleus is supported by observations that archaea and eukarya have similar genes for certain proteins, including histones. Observations that myxobacteria are motile, can form multicellular complexes, and possess kinases and G proteins similar to eukarya, support a bacterial origin for the eukaryotic cell.
A second model proposes that proto-eukaryotic cells evolved from bacteria without an endosymbiotic stage. This model is based on the existence of modern Planctomycetota bacteria that possess a nuclear structure with primitive pores and other compartmentalized membrane structures. A similar proposal states that a eukaryote-like cell, the chronocyte, evolved first and phagocytosed archaea and bacteria to generate the nucleus and the eukaryotic cell.
The most controversial model, known as viral eukaryogenesis, posits that the membrane-bound nucleus, along with other eukaryotic features, originated from the infection of a prokaryote by a virus. The suggestion is based on similarities between eukaryotes and viruses such as linear DNA strands, mRNA capping, and tight binding to proteins (analogizing histones to viral envelopes). One version of the proposal suggests that the nucleus evolved in concert with phagocytosis to form an early cellular "predator". Another variant proposes that eukaryotes originated from early archaea infected by poxviruses, on the basis of observed similarity between the DNA polymerases in modern poxviruses and eukaryotes. It has been suggested that the unresolved question of the evolution of sex could be related to the viral eukaryogenesis hypothesis.
A more recent proposal, the exomembrane hypothesis, suggests that the nucleus instead originated from a single ancestral cell that evolved a second exterior cell membrane; the interior membrane enclosing the original cell then became the nuclear membrane and evolved increasingly elaborate pore structures for passage of internally synthesized cellular components such as ribosomal subunits.
History


The nucleus was the first organelle to be discovered. What is most likely the oldest preserved drawing dates back to the early microscopist Antonie van Leeuwenhoek (1632–1723). He observed a "lumen", the nucleus, in the red blood cells of salmon. Unlike mammalian red blood cells, those of other vertebrates still contain nuclei.
The nucleus was also described by Franz Bauer in 1804 and in more detail in 1831 by Scottish botanist Robert Brown in a talk at the Linnean Society of London. Brown was studying orchids under the microscope when he observed an opaque area, which he called the "areola" or "nucleus", in the cells of the flower's outer layer. He did not suggest a potential function.
In 1838, Matthias Schleiden proposed that the nucleus plays a role in generating cells, thus he introduced the name "cytoblast" ("cell builder"). He believed that he had observed new cells assembling around "cytoblasts". Franz Meyen was a strong opponent of this view, having already described cells multiplying by division and believing that many cells would have no nuclei. The idea that cells can be generated de novo, by the "cytoblast" or otherwise, contradicted work by Robert Remak (1852) and Rudolf Virchow (1855) who decisively propagated the new paradigm that cells are generated solely by cells ("Omnis cellula e cellula"). The function of the nucleus remained unclear.
Between 1877 and 1878, Oscar Hertwig published several studies on the fertilization of sea urchin eggs, showing that the nucleus of the sperm enters the oocyte and fuses with its nucleus. This was the first time it was suggested that an individual develops from a (single) nucleated cell. This was in contradiction to Ernst Haeckel's theory that the complete phylogeny of a species would be repeated during embryonic development, including generation of the first nucleated cell from a "monerula", a structureless mass of primordial protoplasm ("Urschleim"). Therefore, the necessity of the sperm nucleus for fertilization was discussed for quite some time. However, Hertwig confirmed his observation in other animal groups, including amphibians and molluscs. Eduard Strasburger produced the same results for plants in 1884. This paved the way to assign the nucleus an important role in heredity. In 1873, August Weismann postulated the equivalence of the maternal and paternal germ cells for heredity. The function of the nucleus as carrier of genetic information became clear only later, after mitosis was discovered and the Mendelian rules were rediscovered at the beginning of the 20th century; the chromosome theory of heredity was therefore developed.
See also
References
- ^ Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky SL, Darnell J (2004). Molecular Cell Biology (5th ed.). New York: WH Freeman. ISBN 978-0-7167-2672-2.
- Ehrenhofer-Murray AE (June 2004). "Chromatin dynamics at DNA replication, transcription and repair". Review. European Journal of Biochemistry. 271 (12): 2335–49. doi:10.1111/j.1432-1033.2004.04162.x. PMID 15182349.
- Grigoryev SA, Bulynko YA, Popova EY (2006). "The end adjusts the means: heterochromatin remodelling during terminal cell differentiation". Review. Chromosome Research. 14 (1): 53–69. doi:10.1007/s10577-005-1021-6. PMID 16506096. S2CID 6040822.
- Schardin M, Cremer T, Hager HD, Lang M (December 1985). "Specific staining of human chromosomes in Chinese hamster x man hybrid cell lines demonstrates interphase chromosome territories" (PDF). Primary. Human Genetics. 71 (4): 281–7. doi:10.1007/BF00388452. PMID 2416668. S2CID 9261461.
- Lamond AI, Earnshaw WC (April 1998). "Structure and function in the nucleus" (PDF). Review. Science. 280 (5363): 547–53. CiteSeerX 10.1.1.323.5543. doi:10.1126/science.280.5363.547. PMID 9554838.
- Kurz A, Lampel S, Nickolenko JE, Bradl J, Benner A, Zirbel RM, et al. (December 1996). "Active and inactive genes localize preferentially in the periphery of chromosome territories". Primary. The Journal of Cell Biology. 135 (5): 1195–205. doi:10.1083/jcb.135.5.1195. PMC 2121085. PMID 8947544. Archived from the original on 29 September 2007.
- Rothfield NF, Stollar BD (November 1967). "The relation of immunoglobulin class, pattern of anti-nuclear antibody, and complement-fixing antibodies to DNA in sera from patients with systemic lupus erythematosus". Primary. The Journal of Clinical Investigation. 46 (11): 1785–94. doi:10.1172/JCI105669. PMC 292929. PMID 4168731.
- Barned S, Goodman AD, Mattson DH (February 1995). "Frequency of anti-nuclear antibodies in multiple sclerosis". Primary. Neurology. 45 (2): 384–5. doi:10.1212/WNL.45.2.384. PMID 7854544. S2CID 30482028.
- Kume K, Cantwell H, Neumann FR, Jones AW, Snijders AP, Nurse P (May 2017). "A systematic genomic screen implicates nucleocytoplasmic transport and membrane growth in nuclear size control". PLOS Genet. 13 (5): e1006767. doi:10.1371/journal.pgen.1006767. PMC 5436639. PMID 28545058.
- ^ Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P (2015). Molecular Biology of the Cell (6 ed.). New York: Garland Science.
- ^ Lodish HF, Berk A, Kaiser C, Krieger M, Bretscher A, Ploegh H, et al. (2016). Molecular Cell Biology (Eighth ed.). New York: W.H. Freeman. ISBN 978-1-4641-8339-3.
- Shulga N, Mosammaparast N, Wozniak R, Goldfarb DS (May 2000). "Yeast nucleoporins involved in passive nuclear envelope permeability". Primary. The Journal of Cell Biology. 149 (5): 1027–38. doi:10.1083/jcb.149.5.1027. PMC 2174828. PMID 10831607.
- Alberts, Bruce (2019). Essential cell biology (Fifth ed.). New York. p. 242. ISBN 9780393680393.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Pemberton LF, Paschal BM (March 2005). "Mechanisms of receptor-mediated nuclear import and nuclear export". Review. Traffic. 6 (3): 187–98. doi:10.1111/j.1600-0854.2005.00270.x. PMID 15702987. S2CID 172279.
- ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, eds. (2002). "Chapter 4: DNA and Chromosomes". Molecular Biology of the Cell (4th ed.). New York: Garland Science. pp. 191–234. ISBN 978-0-8153-4072-0.
- Stuurman N, Heins S, Aebi U (1998). "Nuclear lamins: their structure, assembly, and interactions". Review. Journal of Structural Biology. 122 (1–2): 42–66. doi:10.1006/jsbi.1998.3987. PMID 9724605.
- Goldman AE, Moir RD, Montag-Lowy M, Stewart M, Goldman RD (November 1992). "Pathway of incorporation of microinjected lamin A into the nuclear envelope". Primary. The Journal of Cell Biology. 119 (4): 725–35. doi:10.1083/jcb.119.4.725. PMC 2289687. PMID 1429833.
- ^ Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP (March 2002). "Nuclear lamins: building blocks of nuclear architecture". Review. Genes & Development. 16 (5): 533–47. doi:10.1101/gad.960502. PMID 11877373.
- Broers JL, Ramaekers FC (2004). "Dynamics of nuclear lamina assembly and disassembly". Review. Symposia of the Society for Experimental Biology (56): 177–92. ISBN 9781134279838. PMID 15565881.
- Moir RD, Yoon M, Khuon S, Goldman RD (December 2000). "Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells". Primary. The Journal of Cell Biology. 151 (6): 1155–68. doi:10.1083/jcb.151.6.1155. PMC 2190592. PMID 11121432.
- Spann TP, Goldman AE, Wang C, Huang S, Goldman RD (February 2002). "Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription". Primary. The Journal of Cell Biology. 156 (4): 603–8. doi:10.1083/jcb.200112047. PMC 2174089. PMID 11854306.
- Mounkes LC, Stewart CL (June 2004). "Aging and nuclear organization: lamins and progeria". Review. Current Opinion in Cell Biology. 16 (3): 322–7. doi:10.1016/j.ceb.2004.03.009. PMID 15145358.
- Hernandez-Verdun D (January 2006). "Nucleolus: from structure to dynamics". Review. Histochemistry and Cell Biology. 125 (1–2): 127–37. doi:10.1007/s00418-005-0046-4. PMID 16328431. S2CID 20769260.
- ^ Lamond AI, Sleeman JE (October 2003). "Nuclear substructure and dynamics". Review. Current Biology. 13 (21): R825-8. Bibcode:2003CBio...13.R825L. doi:10.1016/j.cub.2003.10.012. PMID 14588256. S2CID 16865665.
- Spector DL, Lamond AI (February 2011). "Nuclear speckles". Review. Cold Spring Harbor Perspectives in Biology. 3 (2): a000646. doi:10.1101/cshperspect.a000646. PMC 3039535. PMID 20926517.
- Alexander KA, Coté A, Nguyen SC, Zhang L, Berger SL (March 2021). "p53 mediates target gene association with nuclear speckles for amplified RNA expression". Primary. Molecular Cell. 81 (8): S1097-2765(21)00174-X. doi:10.1016/j.molcel.2021.03.006. PMC 8830378. PMID 33823140. S2CID 233172170.
- ^ Lamond AI, Spector DL (August 2003). "Nuclear speckles: a model for nuclear organelles". Review. Nature Reviews. Molecular Cell Biology. 4 (8): 605–12. doi:10.1038/nrm1172. PMID 12923522. S2CID 6439413.
- Tripathi K, Parnaik VK (September 2008). "Differential dynamics of splicing factor SC35 during the cell cycle" (PDF). Primary. Journal of Biosciences. 33 (3): 345–54. doi:10.1007/s12038-008-0054-3. PMID 19005234. S2CID 6332495. Archived (PDF) from the original on 15 November 2011.
- Tripathi K, Parnaik VK (September 2008). "Differential dynamics of splicing factor SC35 during the cell cycle". Primary. Journal of Biosciences. 33 (3): 345–54. doi:10.1007/s12038-008-0054-3. PMID 19005234. S2CID 6332495.
- Handwerger KE, Gall JG (January 2006). "Subnuclear organelles: new insights into form and function". Review. Trends in Cell Biology. 16 (1): 19–26. doi:10.1016/j.tcb.2005.11.005. PMID 16325406.
- "Cellular component Nucleus speckle". UniProt: UniProtKB. Retrieved 30 August 2013.
- Gall JG, Bellini M, Wu Z, Murphy C (December 1999). "Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes". Primary. Molecular Biology of the Cell. 10 (12): 4385–402. doi:10.1091/mbc.10.12.4385. PMC 25765. PMID 10588665.
- ^ Matera AG, Terns RM, Terns MP (March 2007). "Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs". Review. Nature Reviews. Molecular Cell Biology. 8 (3): 209–20. doi:10.1038/nrm2124. PMID 17318225. S2CID 30268055.
- Bhat P, Chow A, Emert B, et al. (May 2024). "Genome organization around nuclear speckles drives mRNA splicing efficiency". Nature. 629 (5): 1165–1173. doi:10.1038/s41586-024-07429-6. PMC 11164319. PMID 38720076.
- ^ Cioce M, Lamond AI (2005). "Cajal bodies: a long history of discovery". Review. Annual Review of Cell and Developmental Biology. 21: 105–31. doi:10.1146/annurev.cellbio.20.010403.103738. PMID 16212489. S2CID 8807316.
- ^ Pollard TD, Earnshaw WC (2004). Cell Biology. Philadelphia: Saunders. ISBN 978-0-7216-3360-2.
- ^ Matera AG, Frey MR (August 1998). "Coiled bodies and gems: Janus or gemini?". Review. American Journal of Human Genetics. 63 (2): 317–21. doi:10.1086/301992. PMC 1377332. PMID 9683623.
- Matera AG (August 1998). "Of coiled bodies, gems, and salmon". Review. Journal of Cellular Biochemistry. 70 (2): 181–92. doi:10.1002/(sici)1097-4644(19980801)70:2<181::aid-jcb4>3.0.co;2-k. PMID 9671224. S2CID 44941483.
- Navascues J, Berciano MT, Tucker KE, Lafarga M, Matera AG (June 2004). "Targeting SMN to Cajal bodies and nuclear gems during neuritogenesis". Primary. Chromosoma. 112 (8): 398–409. doi:10.1007/s00412-004-0285-5. PMC 1592132. PMID 15164213.
- ^ Lafarga M, Berciano MT, Pena E, Mayo I, Castaño JG, Bohmann D, et al. (August 2002). "Clastosome: a subtype of nuclear body enriched in 19S and 20S proteasomes, ubiquitin, and protein substrates of proteasome". Primary. Molecular Biology of the Cell. 13 (8): 2771–82. CiteSeerX 10.1.1.321.6138. doi:10.1091/mbc.e02-03-0122. PMC 117941. PMID 12181345.
- ^ Dundr M, Misteli T (June 2001). "Functional architecture in the cell nucleus". Review. The Biochemical Journal. 356 (Pt 2): 297–310. doi:10.1042/0264-6021:3560297. PMC 1221839. PMID 11368755.
- Bond CS, Fox AH (September 2009). "Paraspeckles: nuclear bodies built on long noncoding RNA". Review. The Journal of Cell Biology. 186 (5): 637–44. doi:10.1083/jcb.200906113. PMC 2742191. PMID 19720872.
- Goebel HH, Warlo I (January 1997). "Nemaline myopathy with intranuclear rods--intranuclear rod myopathy". Review. Neuromuscular Disorders. 7 (1): 13–9. doi:10.1016/S0960-8966(96)00404-X. PMID 9132135. S2CID 29584217.
- Saunders WS, Cooke CA, Earnshaw WC (November 1991). "Compartmentalization within the nucleus: discovery of a novel subnuclear region". Primary. The Journal of Cell Biology. 115 (4): 919–31. doi:10.1083/jcb.115.4.919. PMC 2289954. PMID 1955462.
- Pombo A, Cuello P, Schul W, Yoon JB, Roeder RG, Cook PR, Murphy S (March 1998). "Regional and temporal specialization in the nucleus: a transcriptionally-active nuclear domain rich in PTF, Oct1 and PIKA antigens associates with specific chromosomes early in the cell cycle". Primary. The EMBO Journal. 17 (6): 1768–78. doi:10.1093/emboj/17.6.1768. PMC 1170524. PMID 9501098.
- Zimber A, Nguyen QD, Gespach C (October 2004). "Nuclear bodies and compartments: functional roles and cellular signalling in health and disease". Review. Cellular Signalling. 16 (10): 1085–104. doi:10.1016/j.cellsig.2004.03.020. PMID 15240004.
- Lallemand-Breitenbach V, de Thé H (May 2010). "PML nuclear bodies". Review. Cold Spring Harbor Perspectives in Biology. 2 (5): a000661. doi:10.1101/cshperspect.a000661. PMC 2857171. PMID 20452955.
- ^ Fox AH, Lamond AI (July 2010). "Paraspeckles". Review. Cold Spring Harbor Perspectives in Biology. 2 (7): a000687. doi:10.1101/cshperspect.a000687. PMC 2890200. PMID 20573717.
- ^ Fox A, Bickmore W (2004). "Nuclear Compartments: Paraspeckles". Nuclear Protein Database. Archived from the original on 10 September 2008. Retrieved 6 March 2007.
- ^ Fox AH, Bond CS, Lamond AI (November 2005). "P54nrb forms a heterodimer with PSP1 that localizes to paraspeckles in an RNA-dependent manner". Primary. Molecular Biology of the Cell. 16 (11): 5304–15. doi:10.1091/mbc.E05-06-0587. PMC 1266428. PMID 16148043.
- Nakagawa S, Yamazaki T, Hirose T (October 2018). "Molecular dissection of nuclear paraspeckles: towards understanding the emerging world of the RNP milieu". Review. Open Biology. 8 (10): 180150. doi:10.1098/rsob.180150. PMC 6223218. PMID 30355755.
- Pisani G, Baron B (December 2019). "Nuclear paraspeckles function in mediating gene regulatory and apoptotic pathways". Review. Non-Coding RNA Research. 4 (4): 128–134. doi:10.1016/j.ncrna.2019.11.002. PMC 7012776. PMID 32072080.
- Kong XN, Yan HX, Chen L, Dong LW, Yang W, Liu Q, et al. (October 2007). "LPS-induced down-regulation of signal regulatory protein {alpha} contributes to innate immune activation in macrophages". Primary. The Journal of Experimental Medicine. 204 (11): 2719–31. doi:10.1084/jem.20062611. PMC 2118489. PMID 17954568.
- ^ Carmo-Fonseca M, Berciano MT, Lafarga M (September 2010). "Orphan nuclear bodies". Review. Cold Spring Harbor Perspectives in Biology. 2 (9): a000703. doi:10.1101/cshperspect.a000703. PMC 2926751. PMID 20610547.
- Sampuda KM, Riley M, Boyd L (April 2017). "Stress induced nuclear granules form in response to accumulation of misfolded proteins in Caenorhabditis elegans". Primary. BMC Cell Biology. 18 (1): 18. doi:10.1186/s12860-017-0136-x. PMC 5395811. PMID 28424053.
- Lehninger AL, Nelson DL, Cox MM (2000). Lehninger principles of biochemistry (3rd ed.). New York: Worth Publishers. ISBN 978-1-57259-931-4.
- Moreno F, Ahuatzi D, Riera A, Palomino CA, Herrero P (February 2005). "Glucose sensing through the Hxk2-dependent signalling pathway". Primary. Biochemical Society Transactions. 33 (Pt 1): 265–8. doi:10.1042/BST0330265. PMID 15667322. S2CID 20647022.
- Görlich D, Kutay U (1999). "Transport between the cell nucleus and the cytoplasm". Review. Annual Review of Cell and Developmental Biology. 15 (1): 607–60. doi:10.1146/annurev.cellbio.15.1.607. PMID 10611974.
- Hozák P, Cook PR (February 1994). "Replication factories". Review. Trends in Cell Biology. 4 (2): 48–52. doi:10.1016/0962-8924(94)90009-4. PMID 14731866.
- Nierhaus KH, Wilson DN (2004). Protein Synthesis and Ribosome Structure: Translating the Genome. Wiley-VCH. ISBN 978-3-527-30638-1.
- Nicolini CA (1997). Genome Structure and Function: From Chromosomes Characterization to Genes Technology. Springer. ISBN 978-0-7923-4565-7.
- Black DL (2003). "Mechanisms of alternative pre-messenger RNA splicing" (PDF). Review. Annual Review of Biochemistry. 72 (1): 291–336. doi:10.1146/annurev.biochem.72.121801.161720. PMID 12626338. S2CID 23576288.
- Watson JD, Baker TA, Bell SP, Gann A, Levine M, Losick R (2004). "Ch9–10". Molecular Biology of the Gene (5th ed.). Peason Benjamin Cummings; CSHL Press. ISBN 978-0-8053-9603-4.
- Cavazza T, Vernos I (2015). "The RanGTP Pathway: From Nucleo-Cytoplasmic Transport to Spindle Assembly and Beyond". Review. Frontiers in Cell and Developmental Biology. 3: 82. doi:10.3389/fcell.2015.00082. PMC 4707252. PMID 26793706.
- Lippincott-Schwartz J (March 2002). "Cell biology: ripping up the nuclear envelope". Commentary. Nature. 416 (6876): 31–2. Bibcode:2002Natur.416...31L. doi:10.1038/416031a. PMID 11882878. S2CID 4431000.
- ^ Boulikas T (1995). "Phosphorylation of transcription factors and control of the cell cycle". Review. Critical Reviews in Eukaryotic Gene Expression. 5 (1): 1–77. PMID 7549180.
- Boettcher B, Barral Y (2013). "The cell biology of open and closed mitosis". Review. Nucleus. 4 (3). Austin, Tex.: 160–5. doi:10.4161/nucl.24676. PMC 3720745. PMID 23644379.
- Steen RL, Collas P (April 2001). "Mistargeting of B-type lamins at the end of mitosis: implications on cell survival and regulation of lamins A/C expression". Primary. The Journal of Cell Biology. 153 (3): 621–6. doi:10.1083/jcb.153.3.621. PMC 2190567. PMID 11331311.
- Böhm I (November 2007). "IgG deposits can be detected in cell nuclei of patients with both lupus erythematosus and malignancy". Primary. Clinical Rheumatology. 26 (11): 1877–82. doi:10.1007/s10067-007-0597-y. PMID 17364135. S2CID 44879431.
- Ressel L (2017). "Nuclear Morphologies". Normal cell morphology in canine and feline cytology: an identification guide. Hoboken, NJ: John Wiley & Sons. p. 6. ISBN 978-1-119-27891-7.
- Skutelsky E, Danon D (June 1970). "Comparative study of nuclear expulsion from the late erythroblast and cytokinesis". Primary. Experimental Cell Research. 60 (3): 427–36. doi:10.1016/0014-4827(70)90536-7. PMID 5422968.
- Torous DK, Dertinger SD, Hall NE, Tometsko CR (February 2000). "Enumeration of micronucleated reticulocytes in rat peripheral blood: a flow cytometric study". Primary. Mutation Research. 465 (1–2): 91–9. doi:10.1016/S1383-5718(99)00216-8. PMID 10708974.
- Hutter KJ, Stöhr M (1982). "Rapid detection of mutagen induced micronucleated erythrocytes by flow cytometry". Primary. Histochemistry. 75 (3): 353–62. doi:10.1007/bf00496738. PMID 7141888. S2CID 28973947.
- Ham BK, Lucas WJ (April 2014). "The angiosperm phloem sieve tube system: a role in mediating traits important to modern agriculture". Journal of Experimental Botany. 65 (7): 1799–816. doi:10.1093/jxb/ert417. PMID 24368503.
- Zettler LA, Sogin ML, Caron DA (October 1997). "Phylogenetic relationships between the Acantharea and the Polycystinea: a molecular perspective on Haeckel's Radiolaria". Primary. Proceedings of the National Academy of Sciences of the United States of America. 94 (21): 11411–6. Bibcode:1997PNAS...9411411A. doi:10.1073/pnas.94.21.11411. PMC 23483. PMID 9326623.
- Horton TR (2006). "The number of nuclei in basidiospores of 63 species of ectomycorrhizal Homobasidiomycetes". Primary. Mycologia. 98 (2): 233–8. doi:10.3852/mycologia.98.2.233. PMID 16894968.
- Adam RD (December 1991). "The biology of Giardia spp". Review. Microbiological Reviews. 55 (4): 706–32. doi:10.1128/MMBR.55.4.706-732.1991. PMC 372844. PMID 1779932.
- Vogt A, Goldman AD, Mochizuki K, Landweber LF (1 August 2013). "Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements". PLOS Genetics. 9 (8): e1003659. doi:10.1371/journal.pgen.1003659. PMC 3731211. PMID 23935529.
- McInnes A, Rennick DM (February 1988). "Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells". Primary. The Journal of Experimental Medicine. 167 (2): 598–611. doi:10.1084/jem.167.2.598. PMC 2188835. PMID 3258008.
- Goldring SR, Roelke MS, Petrison KK, Bhan AK (February 1987). "Human giant cell tumors of bone identification and characterization of cell types". Primary. The Journal of Clinical Investigation. 79 (2): 483–91. doi:10.1172/JCI112838. PMC 424109. PMID 3027126.
- Imanian B, Pombert JF, Dorrell RG, Burki F, Keeling PJ (2012). "Tertiary endosymbiosis in two dinotoms has generated little change in the mitochondrial genomes of their dinoflagellate hosts and diatom endosymbionts". Primary. PLOS ONE. 7 (8): e43763. Bibcode:2012PLoSO...743763I. doi:10.1371/journal.pone.0043763. PMC 3423374. PMID 22916303.
- Pennisi E (August 2004). "Evolutionary biology. The birth of the nucleus". News. Science. 305 (5685): 766–8. doi:10.1126/science.305.5685.766. PMID 15297641. S2CID 83769250.
- Devos DP, Gräf R, Field MC (June 2014). "Evolution of the nucleus". Review. Current Opinion in Cell Biology. 28 (100): 8–15. doi:10.1016/j.ceb.2014.01.004. PMC 4071446. PMID 24508984.
- López-García P, Moreira D (November 2015). "Open Questions on the Origin of Eukaryotes". Review. Trends in Ecology & Evolution. 30 (11): 697–708. doi:10.1016/j.tree.2015.09.005. PMC 4640172. PMID 26455774.
- Hogan CM (2010). "Archaea". In Monosson E, Cleveland C (eds.). Encyclopedia of Earth. Washington, DC.: National Council for Science and the Environment. Archived from the original on 11 May 2011.
- Margulis L (1981). Symbiosis in Cell Evolution. San Francisco: W. H. Freeman and Company. pp. 206–227. ISBN 978-0-7167-1256-5.
- Martin W (December 2005). "Archaebacteria (Archaea) and the origin of the eukaryotic nucleus". Curr Opin Microbiol. 8 (6): 630–7. doi:10.1016/j.mib.2005.10.004. PMID 16242992.
- Bernstein, H., Bernstein, C. (2017). Sexual Communication in Archaea, the Precursor to Eukaryotic Meiosis. In: Witzany, G. (eds) Biocommunication of Archaea. Springer, Cham. https://doi.org/10.1007/978-3-319-65536-9_7
- López-García P, Moreira D (May 2006). "Selective forces for the origin of the eukaryotic nucleus". Review. BioEssays. 28 (5): 525–33. doi:10.1002/bies.20413. PMID 16615090.
- Fuerst JA (2005). "Intracellular compartmentation in planctomycetes". Review. Annual Review of Microbiology. 59: 299–328. doi:10.1146/annurev.micro.59.030804.121258. PMID 15910279.
- Hartman H, Fedorov A (February 2002). "The origin of the eukaryotic cell: a genomic investigation". Primary. Proceedings of the National Academy of Sciences of the United States of America. 99 (3): 1420–5. Bibcode:2002PNAS...99.1420H. doi:10.1073/pnas.032658599. PMC 122206. PMID 11805300.
- Bell PJ (September 2001). "Viral eukaryogenesis: was the ancestor of the nucleus a complex DNA virus?". Comment. Journal of Molecular Evolution. 53 (3): 251–6. Bibcode:2001JMolE..53..251L. doi:10.1007/s002390010215. PMID 11523012. S2CID 20542871.
- Takemura M (May 2001). "Poxviruses and the origin of the eukaryotic nucleus". Primary. Journal of Molecular Evolution. 52 (5): 419–25. Bibcode:2001JMolE..52..419T. doi:10.1007/s002390010171. PMID 11443345. S2CID 21200827.
- Villarreal LP, DeFilippis VR (August 2000). "A hypothesis for DNA viruses as the origin of eukaryotic replication proteins". Primary. Journal of Virology. 74 (15): 7079–84. doi:10.1128/JVI.74.15.7079-7084.2000. PMC 112226. PMID 10888648.
- Bell PJ (November 2006). "Sex and the eukaryotic cell cycle is consistent with a viral ancestry for the eukaryotic nucleus". Primary. Journal of Theoretical Biology. 243 (1): 54–63. Bibcode:2006JThBi.243...54B. doi:10.1016/j.jtbi.2006.05.015. PMID 16846615.
- de Roos AD (2006). "The origin of the eukaryotic cell based on conservation of existing interfaces". Primary. Artificial Life. 12 (4): 513–23. doi:10.1162/artl.2006.12.4.513. PMID 16953783. S2CID 5963228.
- Van Leeuwenhoek A. Opera Omnia, seu Arcana Naturae ope exactissimorum Microscopiorum detecta, experimentis variis comprobata, Epistolis ad varios illustres viros J. Arnold et Delphis, A. Beman, Lugdinum Batavorum [The Works of, or arcana of nature by means of exactissimorum microscopes had been detected and confirmed by a variety of experiments, the Epistles to the various illustrious men of valor J. Arnold and Delphi, A. Beman, Lugdina York 1719-1730] (in Latin). Cited in Gerlach D (2009). Geschichte der Mikroskopie. Frankfurt am Main, Germany: Verlag Harri Deutsch. ISBN 978-3-8171-1781-9.
- Cohen WD (1982). "The cytomorphic system of anucleate non-mammalian erythrocytes". Protoplasma. 113: 23–32. doi:10.1007/BF01283036. S2CID 41287948.
- Harris H (1999). The Birth of the Cell. New Haven: Yale University Press. ISBN 978-0-300-07384-3.
- Brown R (1866). "On the Organs and Mode of Fecundation of Orchidex and Asclepiadea". Miscellaneous Botanical Works I: 511–514.
- ^ Cremer T (1985). Von der Zellenlehre zur Chromosomentheorie. Berlin, Heidelberg, New York, Tokyo: Springer Verlag. ISBN 978-3-540-13987-4. Online Version here
Further reading
- Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP (March 2002). "Nuclear lamins: building blocks of nuclear architecture". Genes & Development. 16 (5): 533–47. doi:10.1101/gad.960502. PMID 11877373.
- A review article about nuclear lamins, explaining their structure and various roles
- Görlich D, Kutay U (1999). "Transport between the cell nucleus and the cytoplasm". Annual Review of Cell and Developmental Biology. 15: 607–60. doi:10.1146/annurev.cellbio.15.1.607. PMID 10611974.
- A review article about nuclear transport, explains the principles of the mechanism, and the various transport pathways
- Lamond AI, Earnshaw WC (April 1998). "Structure and function in the nucleus" (PDF). Science. 280 (5363): 547–53. CiteSeerX 10.1.1.323.5543. doi:10.1126/science.280.5363.547. PMID 9554838.
- A review article about the nucleus, explaining the structure of chromosomes within the organelle, and describing the nucleolus and other subnuclear bodies
- Pennisi E (August 2004). "Evolutionary biology. The birth of the nucleus". Science. 305 (5685): 766–8. doi:10.1126/science.305.5685.766. PMID 15297641. S2CID 83769250.
- A review article about the evolution of the nucleus, explaining a number of different theories
- Pollard TD, Earnshaw WC (2004). Cell Biology. Philadelphia: Saunders. ISBN 978-0-7216-3360-2.
- A university level textbook focusing on cell biology. Contains information on nucleus structure and function, including nuclear transport, and subnuclear domains
External links
Library resources aboutCell nucleus
- "The Nucleus". MBInfo.
- "Learn about the Cell Nucleus". cellnucleus.com. Website covering structure and function of the nucleus from the Department of Oncology at the University of Alberta.
- Bickmore W. "The Nuclear Protein Database". Medical Research Council Human Genetics Unit. Information on nuclear components.
- "The Nucleus Collection". Image & Video Library. The American Society for Cell Biology. Archived from the original on 12 November 2006. contains peer-reviewed still images and video clips that illustrate the nucleus.
- Gall JG, McIntosh JR (eds.). "Nuclear Envelope and Nuclear Import Section". Landmark Papers in Cell Biology. Archived from the original on 17 November 2006. contains digitized commentaries and links to seminal research papers on the nucleus. Published online in the Image & Video Library Archived 10 June 2011 at the Wayback Machine of The American Society for Cell Biology
- "Cytoplasmic patterns generated by human antibodies". AntibodyPatterns.com. Archived from the original on 2 January 2007.
| Structures of the cell / organelles | |
|---|---|
| Endomembrane system | |
| Cytoskeleton | |
| Endosymbionts | |
| Other internal | |
| External | |
| Structures of the cell nucleus / nuclear protein | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Envelope (membrane)/ nuclear lamina | |||||||||||||
| Nucleolus | |||||||||||||
| Other |
| ||||||||||||
| see also nucleus diseases | |||||||||||||