 | |
| Clinical data | |
|---|---|
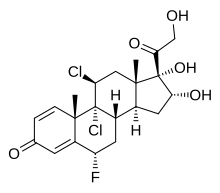
| Other names | 6α-Fluoro-9α,11β-dichloro-16α,17α,21-trihydroxypregna-1,4-diene-3,20-dione |
| Drug class | Corticosteroid; Glucocorticoid |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H25Cl2FO5 |
| Molar mass | 447.32 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Fluclorolone is a synthetic glucocorticoid corticosteroid which was never marketed. The acetonide cyclic ketal of fluclorolone, fluclorolone acetonide, in contrast, has been marketed.
References
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 557–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 448–. ISBN 978-3-88763-075-1.
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |